Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-06-09 , DOI: 10.1016/j.bmc.2017.06.007 Hideki Hirose , Takeshi Yamasaki , Masaki Ogino , Ryo Mizojiri , Yumiko Tamura-Okano , Hiroaki Yashiro , Yo Muraki , Yoshihide Nakano , Jun Sugama , Akito Hata , Shinji Iwasaki , Masanori Watanabe , Tsuyoshi Maekawa , Shizuo Kasai
|
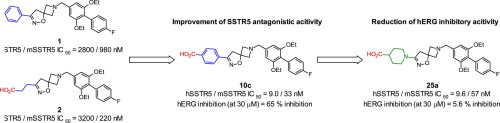
Somatostatin receptor subtype 5 (SSTR5) has emerged as a novel attractive drug target for type 2 diabetes mellitus. Starting from N-benzyl azetidine derivatives 1 and 2 as in-house hit compounds, we explored the introduction of a carboxyl group into the terminal benzene of 1 to enhance SSTR5 antagonistic activity by the combination of the substituents at the 3-position of the isoxazoline. Incorporation of a carboxyl group at the 4-position of the benzene ring resulted in a significant enhancement in potency, however, the 4-benzoic acid derivative 10c exhibited moderate human ether-a-go-go related gene (hERG) inhibitory activity. A subsequent optimization study revealed that replacement of the 4-benzoic acid with an isonipecotic acid dramatically reduced hERG inhibition (5.6% inhibition at 30 μM) by eliminating π-related interaction with hERG K+ channel, which resulted in the identification of 1-(2-((2,6-diethoxy-4′-fluorobiphenyl-4-yl)methyl)-5-oxa-2,6-diazaspiro[3.4]oct-6-en-7-yl)piperidin-4-carboxylic acid 25a (hSSTR5/mSSTR5 IC50 = 9.6/57 nM). Oral administration of 25a in high-fat diet fed C57BL/6J mice augmented insulin secretion in a glucose-dependent manner and lowered blood glucose concentration.
中文翻译:

发现了新型的5-oxa-2,6-二氮杂螺[3.4] oct-6-ene衍生物作为有效,选择性和口服的生长抑素受体亚型5(SSTR5)拮抗剂,可用于治疗2型糖尿病。
生长抑素受体亚型5(SSTR5)已成为2型糖尿病的新型有吸引力的药物靶标。从作为内部命中化合物的N-苄基氮杂环丁烷衍生物1和2开始,我们探索了将羧基引入1的末端苯中以通过异恶唑啉3位上的取代基的组合增强SSTR5拮抗活性的方法。 。在苯环的4位上引入羧基会显着提高效力,但是4-苯甲酸衍生物10c表现出中等程度的人类以太相关基因(hERG)抑制活性。随后的优化研究表明,通过消除与hERG K +通道的π相关相互作用,用异二十二烷酸替换4-苯甲酸显着降低了hERG抑制(在30μM时抑制5.6%),从而鉴定出1-( 2-(((2,6-二乙氧基-4'-氟联苯-4-基)甲基)-5-氧杂-2,6-二氮杂螺[3.4] oct-6-en-7-yl)哌啶-4-羧酸25a(hSSTR5 / mSSTR5 IC 50 = 9.6 / 57 nM)。在高脂饮食喂养的C57BL / 6J小鼠中口服25a可以以葡萄糖依赖性方式增加胰岛素分泌,并降低血糖浓度。






























 京公网安备 11010802027423号
京公网安备 11010802027423号