当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ochracenes A–I, Humulane-Derived Sesquiterpenoids from the Antarctic Fungus Aspergillus ochraceopetaliformis
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-06-09 00:00:00 , DOI: 10.1021/acs.jnatprod.6b00810 Junfeng Wang 1 , Weijun He 1, 2 , Fandong Kong 3 , Xinpeng Tian 1 , Pei Wang 3 , Xiaojiang Zhou 2 , Yonghong Liu 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-06-09 00:00:00 , DOI: 10.1021/acs.jnatprod.6b00810 Junfeng Wang 1 , Weijun He 1, 2 , Fandong Kong 3 , Xinpeng Tian 1 , Pei Wang 3 , Xiaojiang Zhou 2 , Yonghong Liu 1
Affiliation
|
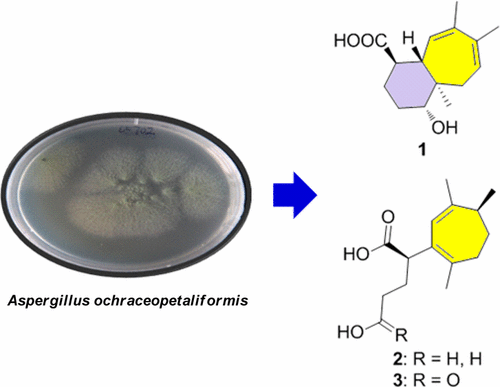
Nine new humulane-derived sesquiterpenoids, ochracenes A–I (1–9), were isolated from the Antarctic fungus Aspergillus ochraceopetaliformis SCSIO 05702. Their structures including absolute configurations were elucidated on the basis of spectroscopic analysis, Mosher’s method, and electronic circular dichroism analysis. Compared with previous humulane-type sesquiterpenoids, ochracenes A–I (1–9) featured novel carbon skeletons with corresponding methyl migration, ring cleavage, and carbon loss. Two unprecedented 8,9-secocyclic sesquiterpenoids (2 and 3) exhibited inhibitory effects on lipopolysaccharide-induced NO release in RAW 264.7 mouse macrophage cell lines with IC50 values of 14.6 ± 0.5 and 18.3 ± 1.7 μM, respectively.
中文翻译:

Ochracenes A–I,来自南极真菌的腐植烷倍半萜类化合物
从南极真菌As曲霉SCSIO 05702中分离出九种新的来自mul草烷的倍半萜类啶类化合物A–I(1 – 9)。在光谱分析,Mosher方法和电子圆二色性分析的基础上阐明了它们的结构,包括绝对构型。与先前的草烷型倍半萜相比,烷A– 1(1–9)具有新颖的碳骨架,具有相应的甲基迁移,环断裂和碳损失。两个史无前例的8,9-半球形倍半萜(2和3)在RAW 264.7小鼠巨噬细胞系中表现出对脂多糖诱导的NO释放的抑制作用,IC 50值分别为14.6±0.5和18.3±1.7μM。
更新日期:2017-06-09
中文翻译:

Ochracenes A–I,来自南极真菌的腐植烷倍半萜类化合物
从南极真菌As曲霉SCSIO 05702中分离出九种新的来自mul草烷的倍半萜类啶类化合物A–I(1 – 9)。在光谱分析,Mosher方法和电子圆二色性分析的基础上阐明了它们的结构,包括绝对构型。与先前的草烷型倍半萜相比,烷A– 1(1–9)具有新颖的碳骨架,具有相应的甲基迁移,环断裂和碳损失。两个史无前例的8,9-半球形倍半萜(2和3)在RAW 264.7小鼠巨噬细胞系中表现出对脂多糖诱导的NO释放的抑制作用,IC 50值分别为14.6±0.5和18.3±1.7μM。















































 京公网安备 11010802027423号
京公网安备 11010802027423号