当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CuH-Catalyzed Regioselective Intramolecular Hydroamination for the Synthesis of Alkyl-Substituted Chiral Aziridines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-06-15 , DOI: 10.1021/jacs.7b04816 Haoxuan Wang 1 , Jeffrey C. Yang 1 , Stephen L. Buchwald 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-06-15 , DOI: 10.1021/jacs.7b04816 Haoxuan Wang 1 , Jeffrey C. Yang 1 , Stephen L. Buchwald 1
Affiliation
|
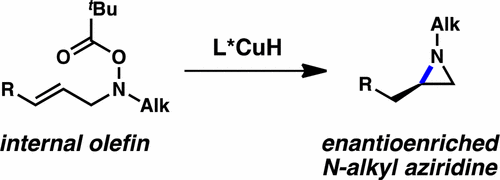
This report details a general and enantioselective means for the synthesis of alkyl-substituted aziridines. This protocol offers a direct route for the synthesis of alkyl-substituted chiral aziridines from achiral starting materials. Readily accessed allylic hydroxylamine esters undergo copper hydride-catalyzed intramolecular hydroamination with a high degree of regio- and enantiocontrol to afford the aziridine products in good to excellent yields in highly enantioenriched form. The utility of the products derived from this method is further demonstrated through derivatization of the chiral aziridine products to obtain a diverse array of functionalized enantioenriched amines.
中文翻译:

用于合成烷基取代手性氮丙啶的 CuH 催化的区域选择性分子内加氢胺化
本报告详细介绍了合成烷基取代氮丙啶的一般和对映选择性方法。该协议为从非手性原料合成烷基取代的手性氮丙啶提供了直接途径。容易获得的烯丙基羟胺酯经过氢化铜催化的分子内加氢胺化,具有高度的区域和对映体控制,以高度对映体富集的形式以良好至极好的收率提供氮丙啶产物。通过手性氮丙啶产品的衍生化,获得了多种功能化的对映体富集胺,进一步证明了该方法衍生的产品的实用性。
更新日期:2017-06-15
中文翻译:

用于合成烷基取代手性氮丙啶的 CuH 催化的区域选择性分子内加氢胺化
本报告详细介绍了合成烷基取代氮丙啶的一般和对映选择性方法。该协议为从非手性原料合成烷基取代的手性氮丙啶提供了直接途径。容易获得的烯丙基羟胺酯经过氢化铜催化的分子内加氢胺化,具有高度的区域和对映体控制,以高度对映体富集的形式以良好至极好的收率提供氮丙啶产物。通过手性氮丙啶产品的衍生化,获得了多种功能化的对映体富集胺,进一步证明了该方法衍生的产品的实用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号