当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can Baird’s and Clar’s Rules Combined Explain Triplet State Energies of Polycyclic Conjugated Hydrocarbons with Fused 4nπ- and (4n + 2)π-Rings?
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-05-23 00:00:00 , DOI: 10.1021/acs.joc.7b00906 Rabia Ayub 1 , Ouissam El Bakouri 2 , Kjell Jorner 1 , Miquel Solà 2 , Henrik Ottosson 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-05-23 00:00:00 , DOI: 10.1021/acs.joc.7b00906 Rabia Ayub 1 , Ouissam El Bakouri 2 , Kjell Jorner 1 , Miquel Solà 2 , Henrik Ottosson 1
Affiliation
|
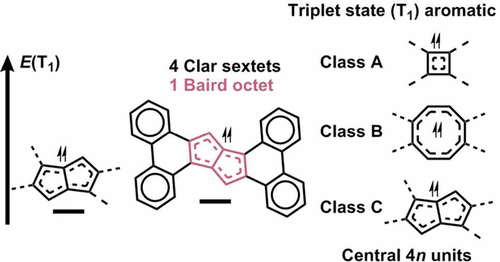
Compounds that can be labeled as “aromatic chameleons” are π-conjugated compounds that are able to adjust their π-electron distributions so as to comply with the different rules of aromaticity in different electronic states. We used quantum chemical calculations to explore how the fusion of benzene rings onto aromatic chameleonic units represented by biphenylene, dibenzocyclooctatetraene, and dibenzo[a,e]pentalene modifies the first triplet excited states (T1) of the compounds. Decreases in T1 energies are observed when going from isomers with linear connectivity of the fused benzene rings to those with cis- or trans-bent connectivities. The T1 energies decreased down to those of the parent (isolated) 4nπ-electron units. Simultaneously, we observe an increased influence of triplet state aromaticity of the central 4n ring as given by Baird’s rule and evidenced by geometric, magnetic, and electron density based aromaticity indices (HOMA, NICS-XY, ACID, and FLU). Because of an influence of triplet state aromaticity in the central 4nπ-electron units, the most stabilized compounds retain the triplet excitation in Baird π-quartets or octets, enabling the outer benzene rings to adapt closed-shell singlet Clar π-sextet character. Interestingly, the T1 energies go down as the total number of aromatic cycles within a molecule in the T1 state increases.
中文翻译:

灿Baird的克拉和的规则进行组合的解释多环共轭烃的三重态的能量与融合4 ñ π-和(4 ñ + 2)π形圈?
可以标记为“芳香变色龙”的化合物是π共轭化合物,它们能够调节其π电子分布,从而在不同的电子状态下符合芳香性的不同规则。我们使用量子化学计算来探索如何将苯环与以联苯,二苯并环辛酸酯和二苯并[ a,e ]戊烯为代表的芳香族chaleleonic单元融合来修饰化合物的第一个三重态激发态(T 1)。当从具有稠合苯环线性连接性的异构体变为具有顺式或反式连接性的异构体时,观察到T 1能量降低。T 1能量下降到母体的能量(隔离的)4n个π电子单位。同时,我们观察到中心4 n环三重态芳香性的影响增加,这由Baird规则给出,并由基于几何,磁性和电子密度的芳香性指数(HOMA,NICS- XY,ACID和FLU)证明。由于在中心4个nπ电子单元中三重态芳香性的影响,最稳定的化合物保留了Bairdπ四重奏或八位位组中的三重态激发,从而使外部苯环能够适应闭壳单重态Clarπ-双峰特征。有趣的是,随着处于T 1状态的分子内芳香环总数的增加,T 1能量下降。
更新日期:2017-06-08
中文翻译:

灿Baird的克拉和的规则进行组合的解释多环共轭烃的三重态的能量与融合4 ñ π-和(4 ñ + 2)π形圈?
可以标记为“芳香变色龙”的化合物是π共轭化合物,它们能够调节其π电子分布,从而在不同的电子状态下符合芳香性的不同规则。我们使用量子化学计算来探索如何将苯环与以联苯,二苯并环辛酸酯和二苯并[ a,e ]戊烯为代表的芳香族chaleleonic单元融合来修饰化合物的第一个三重态激发态(T 1)。当从具有稠合苯环线性连接性的异构体变为具有顺式或反式连接性的异构体时,观察到T 1能量降低。T 1能量下降到母体的能量(隔离的)4n个π电子单位。同时,我们观察到中心4 n环三重态芳香性的影响增加,这由Baird规则给出,并由基于几何,磁性和电子密度的芳香性指数(HOMA,NICS- XY,ACID和FLU)证明。由于在中心4个nπ电子单元中三重态芳香性的影响,最稳定的化合物保留了Bairdπ四重奏或八位位组中的三重态激发,从而使外部苯环能够适应闭壳单重态Clarπ-双峰特征。有趣的是,随着处于T 1状态的分子内芳香环总数的增加,T 1能量下降。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号