当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Facile Method To Prepare Novel Ag2O/Ag2CO3 Three-Dimensional Hollow Hierarchical Structures and Their Water Purification Function
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-05-30 00:00:00 , DOI: 10.1021/acssuschemeng.7b01040 Xiaole Zhao 1 , Yingchun Su 1 , Xiaodong Qi 1 , Xiaojun Han 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-05-30 00:00:00 , DOI: 10.1021/acssuschemeng.7b01040 Xiaole Zhao 1 , Yingchun Su 1 , Xiaodong Qi 1 , Xiaojun Han 1
Affiliation
|
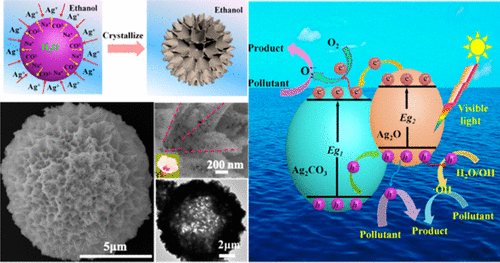
A facile, green, and low-cost method to prepare novel Ag2O/Ag2CO3 three-dimensional hollow flowerlike hierarchical microspheres has been demonstrated. The microspheres were obtained by simple injection of Na2CO3 aqueous solution into AgNO3 ethanol solution. The surface morphology of the microspheres was relatively smooth, trumpet flowerlike hole, and flowerlike shallow hole when the reaction temperature was 8, 25, and 70 °C, respectively. They were assembled by nanoparticles. The microsphere prepared at 25 °C was composed of 86.3% Ag2CO3 and 13.7% Ag2O. The HTEM image indicates that the Ag2CO3 microsphere is wrapped by a thin Ag2O layer, and there is a heterojunction between Ag2CO3 and Ag2O. The study of the formation mechanism demonstrates the first example of using water-soluble Na2CO3 as templates to prepare 3D hierarchical microstructures. The Ag2O/Ag2CO3 microspheres prepared at 25 °C have excellent adsorption and photocatalysis activity for Congo red (CR). The adsorption behavior of the Ag2O/Ag2CO3 microsphere follows the Langmuir isothermal adsorption model. The maximal adsorption capacity of the Ag2O/Ag2CO3 microsphere was about 100.84 mg g–1 for CR in water. Here, 87.5% of CR was removed within 20 min under visible light irradiation at an initial concentration of CR of 80 mg L–1 with a photodegradation rate constant, k, of 0.06814. The radicals (O2·–,·OH) and hole (h+) were proved to be the active species for photocatalyzing CR. The microspheres were reused five times without distinct loss of photocatalysis activity, which indicated that they have stable photocatalysis activity.
中文翻译:

一种新型的Ag 2 O / Ag 2 CO 3三维空心层级结构的制备方法及其水净化功能
已经证明了一种简便,绿色,低成本的方法来制备新颖的Ag 2 O / Ag 2 CO 3三维空心花状分层微球。通过将Na 2 CO 3水溶液简单注入AgNO 3乙醇溶液中获得微球。当反应温度分别为8、25和70°C时,微球的表面形态相对较光滑,喇叭形的花状孔和花状的浅孔。它们由纳米粒子组装。在25°C下制备的微球由86.3%的Ag 2 CO 3和13.7%的Ag 2 O组成。HTEM图像表明Ag 2CO 3微球被薄薄的Ag 2 O层包裹,并且在Ag 2 CO 3和Ag 2 O之间存在异质结。对形成机理的研究证明了使用水溶性Na 2 CO 3作为模板制备第一个例子。准备3D分层微观结构。在25°C下制备的Ag 2 O / Ag 2 CO 3微球对刚果红(CR)具有出色的吸附和光催化活性。Ag 2 O / Ag 2 CO 3的吸附行为微球遵循Langmuir等温吸附模型。Ag 2 O / Ag 2 CO 3微球在水中对CR的最大吸附能力约为100.84 mg g –1。在这里,在可见光照射下,初始CR浓度为80 mg L –1且光降解速率常数k为0.06814时,在20分钟内去除了87.5%的CR 。自由基(O 2 ·–,·OH)和空穴(h +)被证明是光催化CR的活性物种。微球被重复使用了五次而没有明显的光催化活性损失,这表明它们具有稳定的光催化活性。
更新日期:2017-06-28
中文翻译:

一种新型的Ag 2 O / Ag 2 CO 3三维空心层级结构的制备方法及其水净化功能
已经证明了一种简便,绿色,低成本的方法来制备新颖的Ag 2 O / Ag 2 CO 3三维空心花状分层微球。通过将Na 2 CO 3水溶液简单注入AgNO 3乙醇溶液中获得微球。当反应温度分别为8、25和70°C时,微球的表面形态相对较光滑,喇叭形的花状孔和花状的浅孔。它们由纳米粒子组装。在25°C下制备的微球由86.3%的Ag 2 CO 3和13.7%的Ag 2 O组成。HTEM图像表明Ag 2CO 3微球被薄薄的Ag 2 O层包裹,并且在Ag 2 CO 3和Ag 2 O之间存在异质结。对形成机理的研究证明了使用水溶性Na 2 CO 3作为模板制备第一个例子。准备3D分层微观结构。在25°C下制备的Ag 2 O / Ag 2 CO 3微球对刚果红(CR)具有出色的吸附和光催化活性。Ag 2 O / Ag 2 CO 3的吸附行为微球遵循Langmuir等温吸附模型。Ag 2 O / Ag 2 CO 3微球在水中对CR的最大吸附能力约为100.84 mg g –1。在这里,在可见光照射下,初始CR浓度为80 mg L –1且光降解速率常数k为0.06814时,在20分钟内去除了87.5%的CR 。自由基(O 2 ·–,·OH)和空穴(h +)被证明是光催化CR的活性物种。微球被重复使用了五次而没有明显的光催化活性损失,这表明它们具有稳定的光催化活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号