当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective CuH-Catalyzed Hydroacylation Employing Unsaturated Carboxylic Acids as Aldehyde Surrogates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-06-01 00:00:00 , DOI: 10.1021/jacs.7b04937 Yujing Zhou 1 , Jeffrey S. Bandar 1 , Stephen L. Buchwald 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-06-01 00:00:00 , DOI: 10.1021/jacs.7b04937 Yujing Zhou 1 , Jeffrey S. Bandar 1 , Stephen L. Buchwald 1
Affiliation
|
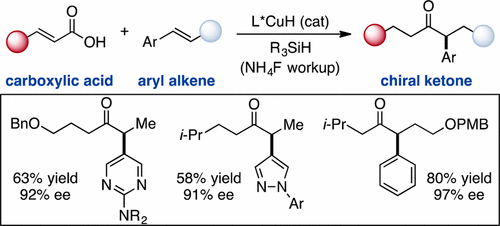
The direct asymmetric copper hydride (CuH)-catalyzed coupling of α,β-unsaturated carboxylic acids to aryl alkenes to access chiral α-aryl dialkyl ketones is reported. A variety of substrate substitution patterns, sensitive functional groups, and heterocycles are tolerated in this reaction, which significantly expands the range of accessible products compared with existing hydroacylation methodology. Although mechanistic studies are ongoing, we propose that CuH-catalyzed silylation of unsaturated acids occurs to access a uniquely effective acyl electrophilic coupling partner.
中文翻译:

使用不饱和羧酸代孕酸酯的对映选择性CuH催化加氢酰化反应
报道了直接不对称氢化铜(CuH)催化的α,β-不饱和羧酸与芳基烯烃的偶联,从而获得手性α-芳基二烷基酮。在该反应中可以耐受多种底物取代方式,敏感的官能团和杂环,与现有的加氢酰化方法相比,这大大扩展了可及产品的范围。尽管正在进行机理研究,但我们建议发生CuH催化的不饱和酸甲硅烷基化反应,以访问独特有效的酰基亲电偶联伙伴。
更新日期:2017-06-07
中文翻译:

使用不饱和羧酸代孕酸酯的对映选择性CuH催化加氢酰化反应
报道了直接不对称氢化铜(CuH)催化的α,β-不饱和羧酸与芳基烯烃的偶联,从而获得手性α-芳基二烷基酮。在该反应中可以耐受多种底物取代方式,敏感的官能团和杂环,与现有的加氢酰化方法相比,这大大扩展了可及产品的范围。尽管正在进行机理研究,但我们建议发生CuH催化的不饱和酸甲硅烷基化反应,以访问独特有效的酰基亲电偶联伙伴。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号