当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phenanthrene Synthesis by Palladium-Catalyzed Benzannulation with o-Bromobenzyl Alcohols through Multiple Carbon–Carbon Bond Formations
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-05-18 00:00:00 , DOI: 10.1021/acs.joc.7b00848 Masayuki Iwasaki 1 , Yasuhiro Araki 1 , Yasushi Nishihara 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-05-18 00:00:00 , DOI: 10.1021/acs.joc.7b00848 Masayuki Iwasaki 1 , Yasuhiro Araki 1 , Yasushi Nishihara 1
Affiliation
|
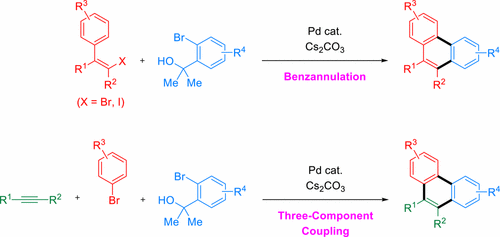
A palladium-catalyzed benzannulation with o-bromobenzyl alcohols enabled the facile construction of phenanthrene skeletons via the sequential multiple carbon–carbon bond formations. A variety of multisubstituted phenanthrenes were synthesized by the reaction of (Z)-β-halostyrenes with o-bromobenzyl alcohols as well as by the three-component coupling of alkynes, aryl bromides, and o-bromobenzyl alcohols. The electron-deficient phosphine ligand played an important role to control the sequential oxidative addition of two different organic halides employed, which realized the selective formation of the desired phenanthrenes in good yields. This synthetic protocol was also applicable to the synthesis of the highly fused polycyclic aromatic hydrocarbons such as tetraphenes.
中文翻译:

邻位溴苄醇经钯催化苯环合反应通过多次碳-碳键形成菲
邻溴苄醇与钯催化的苯环合可以通过连续的多个碳-碳键形成而轻松地构建菲骨架。多种多取代菲的通过的(反应合成Ž)-β-卤代苯乙烯与ö -bromobenzyl醇以及由炔烃组成的三组分偶联,芳基溴化物和ö-溴苄醇。缺电子的膦配体在控制所用的两种不同有机卤化物的顺序氧化加成中起着重要作用,从而实现了以良好的收率选择性形成所需的菲。该合成方案也适用于高度稠合的多环芳族烃如四苯的合成。
更新日期:2017-06-06
中文翻译:

邻位溴苄醇经钯催化苯环合反应通过多次碳-碳键形成菲
邻溴苄醇与钯催化的苯环合可以通过连续的多个碳-碳键形成而轻松地构建菲骨架。多种多取代菲的通过的(反应合成Ž)-β-卤代苯乙烯与ö -bromobenzyl醇以及由炔烃组成的三组分偶联,芳基溴化物和ö-溴苄醇。缺电子的膦配体在控制所用的两种不同有机卤化物的顺序氧化加成中起着重要作用,从而实现了以良好的收率选择性形成所需的菲。该合成方案也适用于高度稠合的多环芳族烃如四苯的合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号