European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-06-03 , DOI: 10.1016/j.ejmech.2017.05.061
Ross P. McGeary , Daniel T.C. Tan , Christopher Selleck , Marcelo Monteiro Pedroso , Hanna E. Sidjabat , Gerhard Schenk
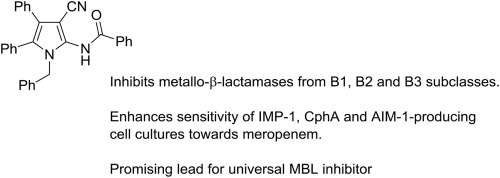
|
A SAR study on derivatives of 2-amino-1-benzyl-4,5-diphenyl-1H-pyrrole-3-carbonitrile 5a revealed that the 3-carbonitrile group, vicinal 4,5-diphenyl and N-benzyl side chains of the pyrrole are important for the inhibitory potencies of these compounds against members representing the three main subclasses of metallo-β-lactamases (MBLs), i.e. IMP-1 (representing the B1 subgroup), CphA (B2) and AIM-1 (B3). Coupling of 5a with a series of acyl chlorides and anhydrides led to the discovery of two N-acylamide derivatives, 10 and 11, as the two most potent IMP-1 inhibitors in this series. However, these compounds are less effective towards CphA and AIM-1. The N-benzoyl derivative of 5a retained potent in vitro activity against each of MBLs tested (with inhibition constants in the low μM range). Importantly, this compound also significantly enhanced the sensitivity of IMP-1, CphA- or AIM-1-producing cell cultures towards meropenem. This compound presents a promising starting point for the development of a universal MBL inhibitor, targeting members of each of the major subgroups of this family of enzymes.
中文翻译:

广谱金属-β-内酰胺酶抑制剂2-氨基吡咯-1-苄基-4,5-二苯基-1H-吡咯-3-腈的构效关系研究及优化
对2-氨基-1-苄基-4,5-二苯基-1 H-吡咯-3-腈5a的衍生物进行的SAR研究表明,3-腈基,邻位的4,5-二苯基和N-苄基侧链吡咯对于这些化合物对代表金属β-内酰胺酶(MBL)的三个主要亚类的成员的抑制能力很重要,即IMP-1(代表B1亚组),CphA(B2)和AIM-1(B3) 。5a与一系列酰氯和酸酐的偶联导致发现两种N-酰基酰胺衍生物10和11,作为该系列中两种最有效的IMP-1抑制剂。但是,这些化合物对CphA和AIM-1的疗效较差。5a的N-苯甲酰基衍生物对每个测试的MBL都具有很强的体外活性(抑制常数在低μM范围内)。重要的是,该化合物还显着增强了产生IMP-1,CphA或AIM-1的细胞培养物对美洛培南的敏感性。该化合物为靶向该酶家族每个主要亚组成员的通用MBL抑制剂的开发提供了一个有希望的起点。































 京公网安备 11010802027423号
京公网安备 11010802027423号