当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remarkably Stereospecific Utilization of ATP α,β-Halomethylene Analogues by Protein Kinases
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-23 00:00:00 , DOI: 10.1021/jacs.7b03266
Feng Ni 1, 2 , Alvin Kung 1, 2 , Yankun Duan 3, 4 , Vivek Shah 1 , Carolina D. Amador 1 , Ming Guo 5 , Xuegong Fan 3 , Lin Chen 1, 4 , Yongheng Chen 5 , Charles E. McKenna 1 , Chao Zhang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-23 00:00:00 , DOI: 10.1021/jacs.7b03266
Feng Ni 1, 2 , Alvin Kung 1, 2 , Yankun Duan 3, 4 , Vivek Shah 1 , Carolina D. Amador 1 , Ming Guo 5 , Xuegong Fan 3 , Lin Chen 1, 4 , Yongheng Chen 5 , Charles E. McKenna 1 , Chao Zhang 1, 2
Affiliation
|
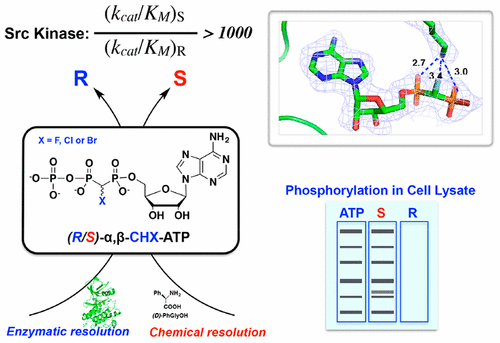
ATP analogues containing a CXY group in place of the α,β-bridging oxygen atom are powerful chemical probes for studying ATP-dependent enzymes. A limitation of such probes has been that conventional synthetic methods generate a mixture of diastereomers when the bridging carbon substitution is nonequivalent (X ≠ Y). We report here a novel method based on derivatization of a bisphosphonate precursor with a d-phenylglycine chiral auxiliary that enables preparation of the individual diastereomers of α,β-CHF-ATP and α,β-CHCl-ATP, which differ only in the configuration at the CHX carbon. When tested on a dozen divergent protein kinases, these individual diastereomers exhibit remarkable diastereospecificity (up to over 1000-fold) in utilization by the enzymes. This high selectivity can be exploited in an enzymatic approach to obtain the otherwise inaccessible diastereomers of α,β-CHBr-ATP. The crystal structure of a tyrosine kinase Src bound to α,β-CHX-ADP establishes the absolute configuration of the CHX carbon and helps clarify the origin of the remarkable diastereospecificity observed. We further synthesized the individual diastereomers of α,β-CHF-γ-thiol-ATP and demonstrated their utility in labeling a wide spectrum of kinase substrates. The novel ATP substrate analogues afforded by these two complementary strategies should have broad application in the study of the structure and function of ATP-dependent enzymes.
中文翻译:

蛋白质激酶对ATPα,β-卤亚甲基类似物的立体定向利用
含有CXY基团代替α,β桥接氧原子的ATP类似物是研究ATP依赖型酶的有力化学探针。这种探针的局限性在于当桥接碳取代不等价时(X≠Y),常规合成方法会生成非对映异构体的混合物。我们在此报告一种基于双膦酸酯前体与d衍生化的新方法-苯基甘氨酸手性助剂,可以制备单独的α,β-CHF-ATP和α,β-CHCl-ATP的非对映异构体,它们的CHX碳原子仅具有不同的构型。当对十二种不同的蛋白激酶进行测试时,这些单独的非对映异构体在酶的利用方面表现出非同寻常的非对映特异性(高达1000倍以上)。这种高选择性可以通过酶促方法获得,以获得原本无法获得的α,β-CHBr-ATP非对映异构体。与α,β-CHX-ADP结合的酪氨酸激酶Src的晶体结构建立了CHX碳的绝对构型,并有助于阐明观察到的非对映特异性的起源。我们进一步合成了α,β-CHF-γ-硫醇-ATP的单个非对映异构体,并证明了它们在标记广泛的激酶底物方面的效用。
更新日期:2017-06-03
中文翻译:

蛋白质激酶对ATPα,β-卤亚甲基类似物的立体定向利用
含有CXY基团代替α,β桥接氧原子的ATP类似物是研究ATP依赖型酶的有力化学探针。这种探针的局限性在于当桥接碳取代不等价时(X≠Y),常规合成方法会生成非对映异构体的混合物。我们在此报告一种基于双膦酸酯前体与d衍生化的新方法-苯基甘氨酸手性助剂,可以制备单独的α,β-CHF-ATP和α,β-CHCl-ATP的非对映异构体,它们的CHX碳原子仅具有不同的构型。当对十二种不同的蛋白激酶进行测试时,这些单独的非对映异构体在酶的利用方面表现出非同寻常的非对映特异性(高达1000倍以上)。这种高选择性可以通过酶促方法获得,以获得原本无法获得的α,β-CHBr-ATP非对映异构体。与α,β-CHX-ADP结合的酪氨酸激酶Src的晶体结构建立了CHX碳的绝对构型,并有助于阐明观察到的非对映特异性的起源。我们进一步合成了α,β-CHF-γ-硫醇-ATP的单个非对映异构体,并证明了它们在标记广泛的激酶底物方面的效用。







































 京公网安备 11010802027423号
京公网安备 11010802027423号