当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NAM-TMS Mechanism of α-Amino Acid N-Carboxyanhydride Polymerization: A DFT Study
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-05-19 00:00:00 , DOI: 10.1021/acs.jpca.7b04278 Tianwen Bai 1 , Jun Ling 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-05-19 00:00:00 , DOI: 10.1021/acs.jpca.7b04278 Tianwen Bai 1 , Jun Ling 1
Affiliation
|
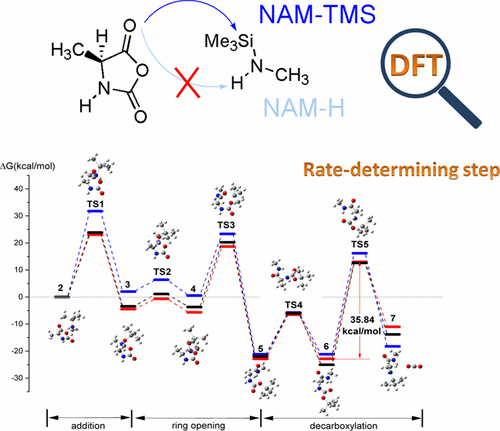
The normal amine mechanism via the proton-transfer route (NAM-H) is widely accepted for the synthesis of polypeptides with nonionic initiators. Besides proton transfer, the trimethylsilyl (TMS) group transfer process has been found in living/controlled polymerization initiated by N-TMS amine in experiments, but the corresponding mechanism has never been proposed. In this work, we employed density functional theory (DFT) with the solvation model to investigate the details of the TMS-transfer mechanism, defined as NAM-TMS, for the ring-opening polymerization of α-amino acid N-carboxyanhydride. The TMS transfer process of NAM-TMS is thermodynamically more favored than the NAM-H mechanism according to the lower addition energy barrier observed. The rate-determining step (RDS) in NAM-TMS is the decarboxylation step, i.e., the release of CO2, rather than carbonyl addition in NAM-H because of the low dipole stable precursor enlarged energy gap of decarboxylation. It is the first calculation evidence supporting decarboxylation as RDS in the NAM mechanism.
中文翻译:

NAM-TMS的α-氨基酸N-羧酰氨基聚合反应机理:DFT研究
通过质子转移途径的正常胺机制(NAM-H)被广泛用于具有非离子引发剂的多肽合成。除质子转移外,在实验中还发现了N -TMS胺引发的活性/受控聚合中的三甲基甲硅烷基(TMS)基团转移过程,但尚未提出相应的机理。在这项工作中,我们采用密度泛函理论(DFT)和溶剂化模型来研究TMS转移机理(称为NAM-TMS)用于α-氨基酸N的开环聚合的细节。-羧基酐。根据观察到的较低的附加能垒,NAM-TMS的TMS转移过程在热力学上比NAM-H机理更受青睐。NAM-TMS中的速率决定步骤(RDS)是脱羧步骤,即释放CO 2,而不是在NAM-H中加入羰基,因为偶极稳定前驱体的低能级扩大了脱羧能隙。这是在NAM机制中支持脱羧作为RDS的第一个计算证据。
更新日期:2017-06-03
中文翻译:

NAM-TMS的α-氨基酸N-羧酰氨基聚合反应机理:DFT研究
通过质子转移途径的正常胺机制(NAM-H)被广泛用于具有非离子引发剂的多肽合成。除质子转移外,在实验中还发现了N -TMS胺引发的活性/受控聚合中的三甲基甲硅烷基(TMS)基团转移过程,但尚未提出相应的机理。在这项工作中,我们采用密度泛函理论(DFT)和溶剂化模型来研究TMS转移机理(称为NAM-TMS)用于α-氨基酸N的开环聚合的细节。-羧基酐。根据观察到的较低的附加能垒,NAM-TMS的TMS转移过程在热力学上比NAM-H机理更受青睐。NAM-TMS中的速率决定步骤(RDS)是脱羧步骤,即释放CO 2,而不是在NAM-H中加入羰基,因为偶极稳定前驱体的低能级扩大了脱羧能隙。这是在NAM机制中支持脱羧作为RDS的第一个计算证据。































 京公网安备 11010802027423号
京公网安备 11010802027423号