当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific Enhancement of Catalytic Activity by a Dicopper Core: Selective Hydroxylation of Benzene to Phenol with Hydrogen Peroxide
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-31 , DOI: 10.1002/anie.201702291 Tomokazu Tsuji 1 , Antonius Andre Zaoputra 1 , Yutaka Hitomi 1 , Kaoru Mieda 2 , Takashi Ogura 2 , Yoshihito Shiota 3 , Kazunari Yoshizawa 3 , Hiroyasu Sato 4 , Masahito Kodera 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-31 , DOI: 10.1002/anie.201702291 Tomokazu Tsuji 1 , Antonius Andre Zaoputra 1 , Yutaka Hitomi 1 , Kaoru Mieda 2 , Takashi Ogura 2 , Yoshihito Shiota 3 , Kazunari Yoshizawa 3 , Hiroyasu Sato 4 , Masahito Kodera 1
Affiliation
|
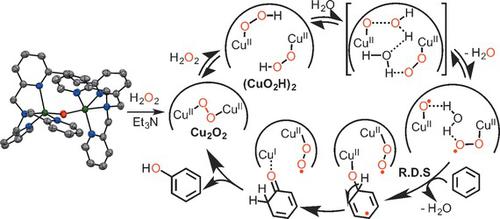
A dicopper(II) complex, stabilized by the bis(tpa) ligand 1,2‐bis[2‐[bis(2‐pyridylmethyl)aminomethyl]‐6‐pyridyl]ethane (6‐hpa), [Cu2(μ‐OH)(6‐hpa)]3+, was synthesized and structurally characterized. This complex catalyzed selective hydroxylation of benzene to phenol using H2O2, thus attaining large turnover numbers (TONs) and high H2O2 efficiency. The TON after 40 hours for the phenol production exceeded 12000 in MeCN at 50 °C under N2, the highest value reported for benzene hydroxylation with H2O2 catalyzed by homogeneous complexes. At 22 % benzene conversion, phenol (95.2 %) and p‐benzoquinone (4.8 %) were produced. The mechanism of H2O2 activation and benzene hydroxylation is proposed.
中文翻译:

Dicopper核的催化活性的特定增强:过氧化氢将苯选择性地羟基化为苯酚
一种双铜(II)络合物,由双(tpa)配体1,2-双[2- [双(2-吡啶基甲基)氨基甲基] -6-吡啶基]乙烷(6-hpa)稳定,[Cu 2(μ- OH)(6-hpa)] 3+,并进行结构表征。该络合物使用H 2 O 2催化苯选择性羟基化为苯酚,从而获得较大的周转数(TONs)和高H 2 O 2效率。在50℃,N 2下的MeCN中,苯酚生产40小时后的TON超过12000 ,这是均相络合物催化H 2 O 2苯羟基化的最高值。苯转化率为22%时,苯酚(95.2%)和p产生了苯醌(4.8%)。提出了H 2 O 2活化和苯羟基化的机理。
更新日期:2017-05-31
中文翻译:

Dicopper核的催化活性的特定增强:过氧化氢将苯选择性地羟基化为苯酚
一种双铜(II)络合物,由双(tpa)配体1,2-双[2- [双(2-吡啶基甲基)氨基甲基] -6-吡啶基]乙烷(6-hpa)稳定,[Cu 2(μ- OH)(6-hpa)] 3+,并进行结构表征。该络合物使用H 2 O 2催化苯选择性羟基化为苯酚,从而获得较大的周转数(TONs)和高H 2 O 2效率。在50℃,N 2下的MeCN中,苯酚生产40小时后的TON超过12000 ,这是均相络合物催化H 2 O 2苯羟基化的最高值。苯转化率为22%时,苯酚(95.2%)和p产生了苯醌(4.8%)。提出了H 2 O 2活化和苯羟基化的机理。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号