当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantum Mechanics Reactive Dynamics Study of Solid Li-Electrode/Li6PS5Cl-Electrolyte Interface
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-05-23 00:00:00 , DOI: 10.1021/acsenergylett.7b00319 Tao Cheng 1 , Boris V. Merinov 1 , Sergey Morozov 2 , William A. Goddard 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-05-23 00:00:00 , DOI: 10.1021/acsenergylett.7b00319 Tao Cheng 1 , Boris V. Merinov 1 , Sergey Morozov 2 , William A. Goddard 1
Affiliation
|
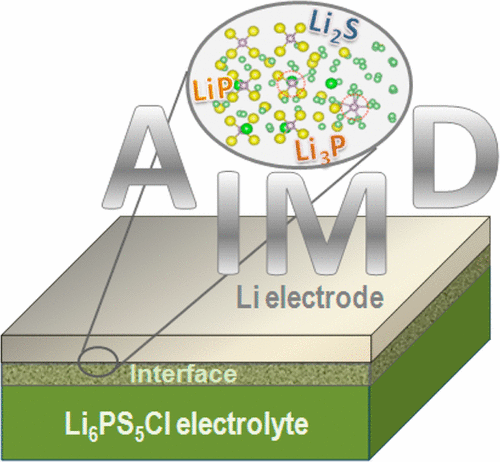
We have performed a theoretical study of the Li/Li6PS5Cl interface and showed the ability of the applied computational methods to properly describe the chemical processes that occur at the interface. After a 500 ps ab initio molecular dynamics simulation, we find that the Li/Li6PS5Cl interface decomposes with formation of multiple phases and that the main decomposition products are Li2S, Li3P, LiCl, and possibly LiP. These findings are in good agreement with reported experimental data. The observed quick decomposition is attributed to the weak bonding between P and S. On the basis of this and earlier obtained experimental results, we conclude that the chemical instability may be an intrinsic problem of P–S-based solid electrolytes when they are in contact with Li-metal. Our results validate the effectiveness of the available computational tools to reach a deeper insight into the evolution of interfacial structures and properties prior to experiment.
中文翻译:

固体Li-电极/ Li 6 PS 5 Cl-电解质界面的量子力学反应动力学研究
我们已经对Li / Li 6 PS 5 Cl界面进行了理论研究,并显示了所应用的计算方法能够正确描述界面处发生的化学过程的能力。经过500 ps从头算的分子动力学模拟,我们发现Li / Li 6 PS 5 Cl界面会分解并形成多相,并且主要分解产物为Li 2 S,Li 3P,LiCl以及可能的LiP。这些发现与报道的实验数据高度吻合。观察到的快速分解归因于P和S之间的弱键合。基于此以及较早获得的实验结果,我们得出结论,化学不稳定可能是P-S基固体电解质接触时的固有问题。与锂金属。我们的结果验证了可用的计算工具的有效性,从而可以在实验之前更深入地了解界面结构和特性的演变。
更新日期:2017-06-01
中文翻译:

固体Li-电极/ Li 6 PS 5 Cl-电解质界面的量子力学反应动力学研究
我们已经对Li / Li 6 PS 5 Cl界面进行了理论研究,并显示了所应用的计算方法能够正确描述界面处发生的化学过程的能力。经过500 ps从头算的分子动力学模拟,我们发现Li / Li 6 PS 5 Cl界面会分解并形成多相,并且主要分解产物为Li 2 S,Li 3P,LiCl以及可能的LiP。这些发现与报道的实验数据高度吻合。观察到的快速分解归因于P和S之间的弱键合。基于此以及较早获得的实验结果,我们得出结论,化学不稳定可能是P-S基固体电解质接触时的固有问题。与锂金属。我们的结果验证了可用的计算工具的有效性,从而可以在实验之前更深入地了解界面结构和特性的演变。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号