Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simple access toward 3-halo- and 3-nitro-pyrazolo[1,5-a]pyrimidines through a one-pot sequence†
RSC Advances ( IF 3.9 ) Pub Date : 2017-05-31 00:00:00 , DOI: 10.1039/c7ra04336h Juan-Carlos Castillo 1, 2, 3, 4, 5 , Hernán-Alejandro Rosero 1, 2, 3, 4, 5 , Jaime Portilla 1, 2, 3, 4, 5
RSC Advances ( IF 3.9 ) Pub Date : 2017-05-31 00:00:00 , DOI: 10.1039/c7ra04336h Juan-Carlos Castillo 1, 2, 3, 4, 5 , Hernán-Alejandro Rosero 1, 2, 3, 4, 5 , Jaime Portilla 1, 2, 3, 4, 5
Affiliation
|
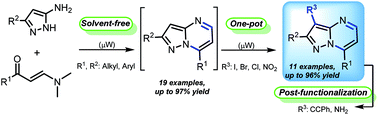
Herein, a regioselective, time-efficient and one-pot route for the synthesis of diversely substituted 3-halo- and 3-nitropyrazolo[1,5-a]pyrimidines in good to excellent yields through a microwave-assisted process is provided. The reaction features a sequential cyclocondensation reaction of β-enaminones with NH-5-aminopyrazoles, followed by a regioselective electrophilic substitution with easily available electrophilic reagents. This methodology is distinguished by its short reaction times, high-yield, operational simplicity, broad substrate scope and pot-economy. Furthermore, these 3-functionalized heterocycles have been successfully used in the synthesis of 3-alkynyl and 3-aminopyrazolo[1,5-a]pyrimidines in yields up to 92%.
中文翻译:

通过一锅法 简单地获得3-卤代和3-硝基吡唑并[1,5- a ]嘧啶†
在此,提供了一种区域选择性,省时和单反应路线,用于通过微波辅助方法以良好至优异的产率合成不同取代的3-卤代和3-硝基吡唑并[1,5- a ]嘧啶。该反应的特征在于β-烯酮与NH-5-氨基吡唑的顺序环缩合反应,然后用容易获得的亲电试剂进行区域选择性亲电取代。该方法的特点是反应时间短,产率高,操作简便,底物范围广和适用范围广。此外,这些3-官能化的杂环已成功地用于合成3-炔基和3-氨基吡唑并[1,5- a ]嘧啶,产率高达92%。
更新日期:2017-05-31
中文翻译:

通过一锅法 简单地获得3-卤代和3-硝基吡唑并[1,5- a ]嘧啶†
在此,提供了一种区域选择性,省时和单反应路线,用于通过微波辅助方法以良好至优异的产率合成不同取代的3-卤代和3-硝基吡唑并[1,5- a ]嘧啶。该反应的特征在于β-烯酮与NH-5-氨基吡唑的顺序环缩合反应,然后用容易获得的亲电试剂进行区域选择性亲电取代。该方法的特点是反应时间短,产率高,操作简便,底物范围广和适用范围广。此外,这些3-官能化的杂环已成功地用于合成3-炔基和3-氨基吡唑并[1,5- a ]嘧啶,产率高达92%。






























 京公网安备 11010802027423号
京公网安备 11010802027423号