当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recognition of EGF-like domains by the Notch-modifying O-fucosyltransferase POFUT1
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-05-22 00:00:00 , DOI: 10.1038/nchembio.2381 Zhijie Li , Kristina Han , John E Pak , Malathy Satkunarajah , Dongxia Zhou , James M Rini
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-05-22 00:00:00 , DOI: 10.1038/nchembio.2381 Zhijie Li , Kristina Han , John E Pak , Malathy Satkunarajah , Dongxia Zhou , James M Rini
|
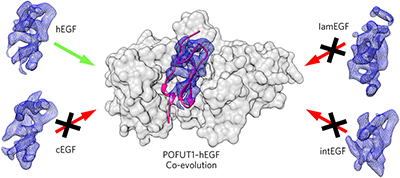
Protein O-fucosyltransferase 1 (POFUT1) fucosylates the epidermal growth factor (EGF)-like domains found in cell-surface and secreted glycoproteins including Notch and its ligands. Although Notch fucosylation is critical for development, and POFUT1 deficiency leads to human disease, how this enzyme binds and catalyzes the fucosylation of its diverse EGF-like domain substrates has not been determined. Reported here is the X-ray crystal structure of mouse POFUT1 in complex with several EGF-like domains, including EGF12 and EGF26 of Notch. Overall shape complementarity, interactions with invariant atoms of the fucosylation motif and flexible segments on POFUT1 all define its EGF-like-domain binding properties. Using large-scale structural and sequence analysis, we also show that POFUT1 binds EGF-like domains of the hEGF type and that the highly correlated presence of POFUT1 and fucosylatable hEGFs has accompanied animal evolution.
中文翻译:

Notch修饰的O-岩藻糖基转移酶POFUT1对EGF样结构域的识别
蛋白质O-岩藻糖基转移酶1(POFUT1)岩藻糖基化在细胞表面和分泌的糖蛋白(包括Notch及其配体)中发现的表皮生长因子(EGF)样结构域。虽然Notch岩藻糖基化对于发育至关重要,并且POFUT1缺乏会导致人类疾病,但尚未确定该酶如何结合和催化其多种EGF样结构域底物的岩藻糖基化。此处报道的是小鼠POFUT1的X射线晶体结构,该结构具有多个EGF样结构域,包括Notch的EGF12和EGF26。整体形状的互补性,与岩藻糖基化基序的不变原子的相互作用以及POFUT1上的柔性链段均定义了其EGF样结构域结合特性。使用大规模的结构和序列分析,
更新日期:2017-05-29
中文翻译:

Notch修饰的O-岩藻糖基转移酶POFUT1对EGF样结构域的识别
蛋白质O-岩藻糖基转移酶1(POFUT1)岩藻糖基化在细胞表面和分泌的糖蛋白(包括Notch及其配体)中发现的表皮生长因子(EGF)样结构域。虽然Notch岩藻糖基化对于发育至关重要,并且POFUT1缺乏会导致人类疾病,但尚未确定该酶如何结合和催化其多种EGF样结构域底物的岩藻糖基化。此处报道的是小鼠POFUT1的X射线晶体结构,该结构具有多个EGF样结构域,包括Notch的EGF12和EGF26。整体形状的互补性,与岩藻糖基化基序的不变原子的相互作用以及POFUT1上的柔性链段均定义了其EGF样结构域结合特性。使用大规模的结构和序列分析,






























 京公网安备 11010802027423号
京公网安备 11010802027423号