当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic enantioselective synthesis of atropisomeric biaryls by a cation-directed O-alkylation
Nature Chemistry ( IF 19.2 ) Pub Date : 2017-01-23 00:00:00 , DOI: 10.1038/nchem.2710 John D. Jolliffe , Roly J. Armstrong , Martin D. Smith
Nature Chemistry ( IF 19.2 ) Pub Date : 2017-01-23 00:00:00 , DOI: 10.1038/nchem.2710 John D. Jolliffe , Roly J. Armstrong , Martin D. Smith
|
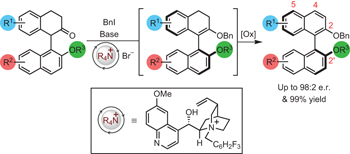
Axially chiral biaryls, as exemplified by 1,1′-bi-2-naphthol (BINOL), are key components of catalysts, natural products and medicines. These materials are synthesized conventionally in enantioenriched form through metal-mediated cross coupling, de novo construction of an aromatic ring, point-to-axial chirality transfer or an atropselective transformation of an existing biaryl. Here, we report a highly enantioselective organocatalytic method for the synthesis of atropisomeric biaryls by a cation-directed O-alkylation. Treatment of racemic 1-aryl-2-tetralones with a chiral quinidine-derived ammonium salt under basic conditions in the presence of an alkylating agent leads to atropselective O-alkylation with e.r. up to 98:2. Oxidation with DDQ gives access to C2-symmetric and non-symmetric BINOL derivatives without compromising e.r. We propose that the chiral ammonium counterion differentiates between rapidly equilibrating atropisomeric enolates, leading to highly atropselective O-alkylation. This dynamic kinetic resolution process offers a general approach to the synthesis of enantioenriched atropisomeric materials.
中文翻译:

阳离子定向的O-烷基化催化对映异构联芳基的催化对映选择性合成
轴向手性联芳基,以1,1'-联-2-萘酚(BINOL)为例,是催化剂,天然产物和药物的关键成分。这些材料通常通过金属介导的交叉偶联,芳环的从头构建,现有联芳基的点对轴手性转移或对映选择性转化而以对映体富集的形式合成。在这里,我们报告了一种高度对映体选择性的有机催化方法,用于通过阳离子定向的O-烷基化反应合成阻转异构联芳基。在碱性条件下,在烷基化剂的存在下,用手性奎尼丁衍生的铵盐处理外消旋的1-芳基-2-四氢萘酮,导致对映选择性的O-烷基化,er最高为98:2。用DDQ氧化可访问不损害er的C 2对称和不对称BINOL衍生物我们建议手性铵抗衡离子在快速平衡的阻转异构烯醇酸酯之间进行区分,从而导致高度阻转性的O-烷基化。这种动态动力学拆分过程为合成富含对映体的阻转异构材料提供了一种通用方法。
更新日期:2017-05-29
中文翻译:

阳离子定向的O-烷基化催化对映异构联芳基的催化对映选择性合成
轴向手性联芳基,以1,1'-联-2-萘酚(BINOL)为例,是催化剂,天然产物和药物的关键成分。这些材料通常通过金属介导的交叉偶联,芳环的从头构建,现有联芳基的点对轴手性转移或对映选择性转化而以对映体富集的形式合成。在这里,我们报告了一种高度对映体选择性的有机催化方法,用于通过阳离子定向的O-烷基化反应合成阻转异构联芳基。在碱性条件下,在烷基化剂的存在下,用手性奎尼丁衍生的铵盐处理外消旋的1-芳基-2-四氢萘酮,导致对映选择性的O-烷基化,er最高为98:2。用DDQ氧化可访问不损害er的C 2对称和不对称BINOL衍生物我们建议手性铵抗衡离子在快速平衡的阻转异构烯醇酸酯之间进行区分,从而导致高度阻转性的O-烷基化。这种动态动力学拆分过程为合成富含对映体的阻转异构材料提供了一种通用方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号