Electrochimica Acta ( IF 5.5 ) Pub Date : 2017-05-26 , DOI: 10.1016/j.electacta.2017.05.158 Vanessa Ramírez-Delgado , Alia Méndez-Albores , Annia Galano , Felipe J. González
|
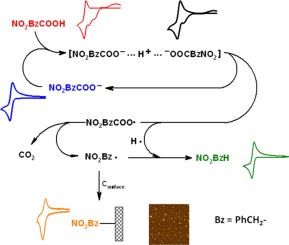
The mechanism of the spontaneous decomposition of a mixture of 4-nitrophenylacetic acid (NO2BzCOOH) with its respective tetrabutylammonium carboxylate in acetonitrile (NO2BzCOO−) was analyzed using cyclic voltammetry, atomic force microscopy, nuclear magnetic resonance and electronic structure calculations. This reaction is favoured by the high acidity of the carboxylic acid and occurs following a decarboxylation loop where the carboxylate anion has the function of reductant whereas the acidic proton of the carboxylic acid acts as oxidant. This redox reaction allows the generation of hydrogen as well as an acyloxy radical, that after cleavage, yields 4-nitrobenzyl radicals that have the possibility to react either in solution to produce 4-nitrotoluene or react with a carbon surface to produce a covalently modified layer with 4-nitrobenzyl groups.
中文翻译:

强羧酸-羧酸盐混合物的自发脱羧作用,并利用碳表面捕获释放的自由基
的4-硝基苯基乙酸(NO的混合物的自发分解的机理2 BzCOOH)与其相应的四丁基铵羧酸盐在乙腈(NO 2 BzCOO -使用循环伏安法,原子力显微镜,核磁共振和电子结构计算来分析)。羧酸的高酸度有利于该反应,并且该反应在脱羧环之后发生,其中羧酸根阴离子具有还原剂的功能,而羧酸的酸性质子充当氧化剂。该氧化还原反应允许产生氢以及酰氧基,其在裂解后产生4-硝基苄基自由基,该4-硝基苄基自由基可能在溶液中反应生成4-硝基甲苯或与碳表面反应生成共价改性层具有4-硝基苄基。

































 京公网安备 11010802027423号
京公网安备 11010802027423号