当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Single Lipase-Catalysed One-Pot Protocol Combining Aminolysis Resolution and Aza-Michael Addition: An Easy and Efficient Way to Synthesise β-Amino Acid Esters
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2015-07-17 , DOI: 10.1002/ejoc.201500760 Fan Xu , Qiongsi Wu , Xiaoyang Chen , Xianfu Lin , Qi Wu
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2015-07-17 , DOI: 10.1002/ejoc.201500760 Fan Xu , Qiongsi Wu , Xiaoyang Chen , Xianfu Lin , Qi Wu
|
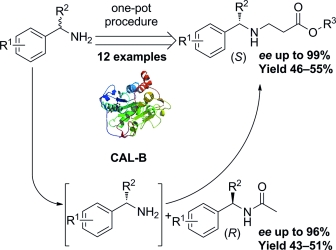
A novel one-pot protocol combining aza-Michael addition and aminolysis resolution was developed to obtain chiral β-amino acid esters with lipase B from Candida antarctica (CAL-B) as the only catalyst. This method is conducted under mild reaction conditions and is very easy to handle. After a series of detailed optimization studies, ten racemic aromatic or aliphatic amines were subjected to this one-pot procedure, and twelve chiral β-amino acid esters and ten chiral amides were successfully synthesised with excellent ee values in theoretical yields. Scaled-up procedures also worked without apparent reduction in reaction rate or enantioselectivity, which makes this method suitable for large-scale production of chiral β-amino acid esters.
中文翻译:

单一脂肪酶催化的一锅法结合氨解分解和 Aza-Michael 加成:合成 β-氨基酸酯的简单有效方法
开发了一种结合 aza-Michael 加成和氨解拆分的新型一锅法,以来自南极念珠菌 (CAL-B) 的脂肪酶 B 作为唯一催化剂获得手性 β-氨基酸酯。该方法在温和的反应条件下进行,非常容易操作。经过一系列详细的优化研究,10 种外消旋芳香族或脂肪族胺经过此一锅法处理,成功合成了 12 种手性 β-氨基酸酯和 10 种手性酰胺,其 ee 值在理论产率下具有优异的值。放大程序也没有明显降低反应速率或对映选择性,这使得该方法适用于手性 β-氨基酸酯的大规模生产。
更新日期:2015-07-17
中文翻译:

单一脂肪酶催化的一锅法结合氨解分解和 Aza-Michael 加成:合成 β-氨基酸酯的简单有效方法
开发了一种结合 aza-Michael 加成和氨解拆分的新型一锅法,以来自南极念珠菌 (CAL-B) 的脂肪酶 B 作为唯一催化剂获得手性 β-氨基酸酯。该方法在温和的反应条件下进行,非常容易操作。经过一系列详细的优化研究,10 种外消旋芳香族或脂肪族胺经过此一锅法处理,成功合成了 12 种手性 β-氨基酸酯和 10 种手性酰胺,其 ee 值在理论产率下具有优异的值。放大程序也没有明显降低反应速率或对映选择性,这使得该方法适用于手性 β-氨基酸酯的大规模生产。































 京公网安备 11010802027423号
京公网安备 11010802027423号