当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalization of Ruthenium(II)(η6 -p-cymene)(3-hydroxy-2-pyridone) Complexes with (Thio)Morpholine: Synthesis and Bioanalytical Studies.
ChemPlusChem ( IF 3.0 ) Pub Date : 2017-04-05 , DOI: 10.1002/cplu.201700050 Muhammad Hanif 1, 2 , Samuel M Meier 2, 3 , Zenita Adhireksan 4 , Helena Henke 2 , Sanela Martic 5 , Sanam Movassaghi 1 , Mahmoud Labib 6 , Wolfgang Kandioller 2, 7 , Stephen M F Jamieson 8 , Michaela Hejl 2 , Michael A Jakupec 2, 7 , Heinz-Bernhard Kraatz 9, 10 , Curt A Davey 4, 11 , Bernhard K Keppler 2, 7 , Christian G Hartinger 1, 2
ChemPlusChem ( IF 3.0 ) Pub Date : 2017-04-05 , DOI: 10.1002/cplu.201700050 Muhammad Hanif 1, 2 , Samuel M Meier 2, 3 , Zenita Adhireksan 4 , Helena Henke 2 , Sanela Martic 5 , Sanam Movassaghi 1 , Mahmoud Labib 6 , Wolfgang Kandioller 2, 7 , Stephen M F Jamieson 8 , Michaela Hejl 2 , Michael A Jakupec 2, 7 , Heinz-Bernhard Kraatz 9, 10 , Curt A Davey 4, 11 , Bernhard K Keppler 2, 7 , Christian G Hartinger 1, 2
Affiliation
|
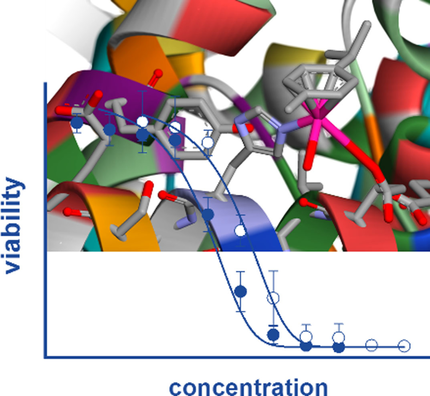
Hydroxypyr(id)ones constitute an emerging platform for the design of drug molecules, owing to their favorable biocompatibility and toxicity profiles. Herein, [RuII (η6 -p-cymene)] complexes with 3-hydroxy-2-pyridinone functionalized with morpholine and thiomorpholine, as a means often used in medicinal chemistry to alter the physicochemical properties of drug compounds, are reported. The compounds underwent hydrolysis of the Ru-Cl bond and the aqua species were stable for up to 48 h in aqueous solution, as observed by 1 H NMR spectroscopy and ESI-MS. The compounds formed adducts with amino acids and proteins through cleavage of the pyridinone ligand. Binding experiments to the nucleosome core particle by means of X-ray crystallography revealed similar reactivity and exclusive binding to histidine moieties of the histone proteins. Preliminary cyclin-dependent kinase 2 (CDK2)/cyclin A kinase inhibitory studies revealed promising activity similar to that of structurally related organometallic compounds.
中文翻译:

钌(II)(η6-对-cymene)(3-羟基-2-吡啶酮)配合物与(硫代)吗啉的功能化:合成和生物分析研究。
羟基吡啶酮由于其良好的生物相容性和毒性特征而构成了设计药物分子的新兴平台。在本文中,报道了用吗啉和硫代吗啉官能化的具有3-羟基-2-吡啶酮的[RuII(η6-对-cymene)]复合物,其经常用于药物化学中以改变药物化合物的理化性质。通过1 H NMR光谱和ESI-MS观察,这些化合物经历了Ru-Cl键的水解,并且水族化合物在水溶液中稳定长达48小时。这些化合物通过吡啶酮配体的裂解与氨基酸和蛋白质形成加合物。通过X射线晶体学对核小体核心颗粒的结合实验显示出相似的反应性,并且与组蛋白的组氨酸部分完全结合。
更新日期:2017-04-05
中文翻译:

钌(II)(η6-对-cymene)(3-羟基-2-吡啶酮)配合物与(硫代)吗啉的功能化:合成和生物分析研究。
羟基吡啶酮由于其良好的生物相容性和毒性特征而构成了设计药物分子的新兴平台。在本文中,报道了用吗啉和硫代吗啉官能化的具有3-羟基-2-吡啶酮的[RuII(η6-对-cymene)]复合物,其经常用于药物化学中以改变药物化合物的理化性质。通过1 H NMR光谱和ESI-MS观察,这些化合物经历了Ru-Cl键的水解,并且水族化合物在水溶液中稳定长达48小时。这些化合物通过吡啶酮配体的裂解与氨基酸和蛋白质形成加合物。通过X射线晶体学对核小体核心颗粒的结合实验显示出相似的反应性,并且与组蛋白的组氨酸部分完全结合。






























 京公网安备 11010802027423号
京公网安备 11010802027423号