Synthesis ( IF 2.2 ) Pub Date : 2015-07-16 , DOI: 10.1055/s-0034-1381010 Quanrui Wang , Chao Wang , Caifei Tang , Zhiming Li
|
Abstract
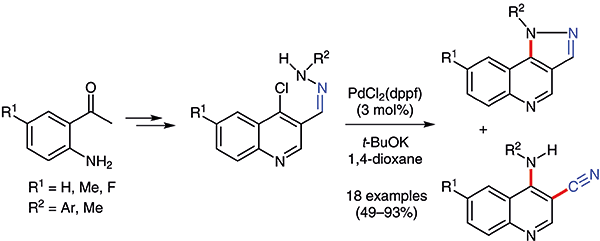
Novel 1H-pyrazolo[4,3-c]quinolines and 4-anilinoquinoline-3-carbonitriles were prepared from readily accessible 4-chloroquinoline-3-carbaldehyde hydrazones by palladium-catalyzed intramolecular C–N bond formation and concurrent hydrazine N–N bond fission. These two classes of functionalized quinolines were chromatographically separable, and both are of biological interest. The ratio of the two products was found to be temperature-dependent; by changing the reaction temperature, either the pyrazoloquinoline or the anilinoquinolinecarbonitrile can become the dominant product.
Novel 1H-pyrazolo[4,3-c]quinolines and 4-anilinoquinoline-3-carbonitriles were prepared from readily accessible 4-chloroquinoline-3-carbaldehyde hydrazones by palladium-catalyzed intramolecular C–N bond formation and concurrent hydrazine N–N bond fission. These two classes of functionalized quinolines were chromatographically separable, and both are of biological interest. The ratio of the two products was found to be temperature-dependent; by changing the reaction temperature, either the pyrazoloquinoline or the anilinoquinolinecarbonitrile can become the dominant product.
中文翻译:

钯催化从4-氯喹啉-3-甲醛中合成1H-吡唑并[4,3-c]喹啉和4-茴香基喹啉-3-甲腈
摘要
新型1 H-吡唑并[4,3- c ]喹啉和4-苯胺基喹啉-3-腈是由易于获得的4-氯喹啉-3-甲醛hydr通过钯催化的分子内C–N键形成和同时形成的肼N–N制备的键裂变。这两类官能化的喹啉在色谱上是可分离的,并且都具有生物学意义。发现两种产物的比例是温度依赖性的。通过改变反应温度,吡唑并喹啉或苯并喹啉腈可以成为主要产物。
新型1 H-吡唑并[4,3- c ]喹啉和4-苯胺基喹啉-3-腈是由易于获得的4-氯喹啉-3-甲醛hydr通过钯催化的分子内C–N键形成和同时形成的肼N–N制备的键裂变。这两类官能化的喹啉在色谱上是可分离的,并且都具有生物学意义。发现两种产物的比例是温度依赖性的。通过改变反应温度,吡唑并喹啉或苯并喹啉腈可以成为主要产物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号