当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Methyl-6,7-dihydro-4H-triazolo[4,5-c]pyridine-Based P2X7 Receptor Antagonists: Optimization of Pharmacokinetic Properties Leading to the Identification of a Clinical Candidate
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-11 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00408
Michael A. Letavic 1 , Brad M. Savall 1 , Brett D. Allison 1 , Leah Aluisio 1 , Jose Ignacio Andres 2 , Meri De Angelis 2 , Hong Ao 1 , Derek A. Beauchamp 3 , Pascal Bonaventure 1 , Stewart Bryant 3 , Nicholas I. Carruthers 1 , Marc Ceusters 4 , Kevin J. Coe 1 , Curt A. Dvorak 1 , Ian C. Fraser 1 , Christine F. Gelin 1 , Tatiana Koudriakova 1 , Jimmy Liang 1 , Brian Lord 1 , Timothy W. Lovenberg 1 , Monicah A. Otieno 3 , Freddy Schoetens 1 , Devin M. Swanson 1 , Qi Wang 1 , Alan D. Wickenden 1 , Anindya Bhattacharya 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-11 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00408
Michael A. Letavic 1 , Brad M. Savall 1 , Brett D. Allison 1 , Leah Aluisio 1 , Jose Ignacio Andres 2 , Meri De Angelis 2 , Hong Ao 1 , Derek A. Beauchamp 3 , Pascal Bonaventure 1 , Stewart Bryant 3 , Nicholas I. Carruthers 1 , Marc Ceusters 4 , Kevin J. Coe 1 , Curt A. Dvorak 1 , Ian C. Fraser 1 , Christine F. Gelin 1 , Tatiana Koudriakova 1 , Jimmy Liang 1 , Brian Lord 1 , Timothy W. Lovenberg 1 , Monicah A. Otieno 3 , Freddy Schoetens 1 , Devin M. Swanson 1 , Qi Wang 1 , Alan D. Wickenden 1 , Anindya Bhattacharya 1
Affiliation
|
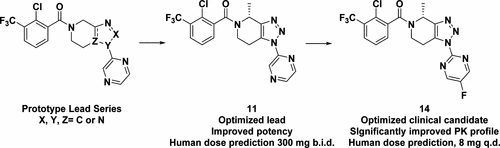
The synthesis and preclinical characterization of novel 4-(R)-methyl-6,7-dihydro-4H-triazolo[4,5-c]pyridines that are potent and selective brain penetrant P2X7 antagonists are described. Optimization efforts based on previously disclosed unsubstituted 6,7-dihydro-4H-triazolo[4,5-c]pyridines, methyl substituted 5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyrazines, and several other series lead to the identification of a series of 4-(R)-methyl-6,7-dihydro-4H-triazolo[4,5-c]pyridines that are selective P2X7 antagonists with potency at the rodent and human P2X7 ion channels. These novel P2X7 antagonists have suitable physicochemical properties, and several analogs have an excellent pharmacokinetic profile, good partitioning into the CNS and show robust in vivo target engagement after oral dosing. Improvements in metabolic stability led to the identification of JNJ-54175446 (14) as a candidate for clinical development. The drug discovery efforts and strategies that resulted in the identification of the clinical candidate are described herein.
中文翻译:

4-甲基-6,7-二氢-4 H-三唑并[4,5 - c ]吡啶基P2X7受体拮抗剂:药代动力学特性的优化,导致临床候选物的鉴定
描述了新型的4-(R)-甲基-6,7-二氢-4 H-三唑并[4,5- c ]吡啶的合成和临床前表征,它们是有效的和选择性的脑渗透剂P2X7拮抗剂。基于先前公开的未取代的6,7-二氢-4 H-三唑[4,5- c ]吡啶,甲基取代的5,6,7,8-四氢[1,2,4]三唑[4,3-]进行的优化工作a ]吡嗪和其他几个系列的鉴定,鉴定出一系列4-(R)-甲基-6,7-二氢-4 H-三唑[4,5- c]吡啶是选择性的P2X7拮抗剂,在啮齿动物和人P2X7离子通道上具有效力。这些新颖的P2X7拮抗剂具有合适的理化性质,几种类似物具有出色的药代动力学特征,可很好地分配到CNS中,并在口服给药后显示出强大的体内靶标参与性。代谢稳定性的改善导致JNJ-54175446(14)被鉴定为临床开发的候选药物。本文描述了导致鉴定临床候选者的药物发现努力和策略。
更新日期:2017-05-26
中文翻译:

4-甲基-6,7-二氢-4 H-三唑并[4,5 - c ]吡啶基P2X7受体拮抗剂:药代动力学特性的优化,导致临床候选物的鉴定
描述了新型的4-(R)-甲基-6,7-二氢-4 H-三唑并[4,5- c ]吡啶的合成和临床前表征,它们是有效的和选择性的脑渗透剂P2X7拮抗剂。基于先前公开的未取代的6,7-二氢-4 H-三唑[4,5- c ]吡啶,甲基取代的5,6,7,8-四氢[1,2,4]三唑[4,3-]进行的优化工作a ]吡嗪和其他几个系列的鉴定,鉴定出一系列4-(R)-甲基-6,7-二氢-4 H-三唑[4,5- c]吡啶是选择性的P2X7拮抗剂,在啮齿动物和人P2X7离子通道上具有效力。这些新颖的P2X7拮抗剂具有合适的理化性质,几种类似物具有出色的药代动力学特征,可很好地分配到CNS中,并在口服给药后显示出强大的体内靶标参与性。代谢稳定性的改善导致JNJ-54175446(14)被鉴定为临床开发的候选药物。本文描述了导致鉴定临床候选者的药物发现努力和策略。







































 京公网安备 11010802027423号
京公网安备 11010802027423号