当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
High-Affinity RGD-Knottin Peptide as a New Tool for Rapid Evaluation of the Binding Strength of Unlabeled RGD-Peptides to αvβ3, αvβ5, and α5β1 Integrin Receptors
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-05-11 00:00:00 , DOI: 10.1021/acs.analchem.7b00554 Dominik Bernhagen 1 , Laura De Laporte 2 , Peter Timmerman 1, 3
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-05-11 00:00:00 , DOI: 10.1021/acs.analchem.7b00554 Dominik Bernhagen 1 , Laura De Laporte 2 , Peter Timmerman 1, 3
Affiliation
|
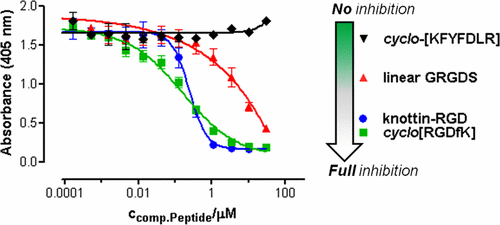
We describe a highly sensitive competition ELISA to measure integrin-binding of RGD-peptides in high-throughput without using cells, ECM-proteins, or antibodies. The assay measures (nonlabeled) RGD-peptides’ ability to inhibit binding of a biotinylated “knottin”-RGD peptide to surface-immobilized integrins and, thus, enables quantification of the binding strength of high-, medium-, and low-affinity RGD-binders. We introduced the biotinylated knottin-RGD peptide instead of biotinylated cyclo[RGDfK] (as reported by Piras et al.), as integrin-binding was much stronger and clearly detectable for all three integrins. In order to maximize sensitivity and cost-efficiency, we first optimized several parameters, such as integrin-immobilization levels, knottin-RGD concentration, buffer compositions, type of detection tag (biotin, His- or cMyc-tag), and spacer length. We thereby identified two key factors, that is, (i) the critical spacer length (longer than Gly) and (ii) the presence of Ca2+ and Mg2+ in all incubation and washing buffers. Binding of knottin-RGD peptide was strongest for αvβ3 but also detectable for both αvβ5 and α5β1, while binding of biotinylated cyclo[RGDfK] was very weak and only detectable for αvβ3. For assay validation, we finally determined IC50 values for three unlabeled peptides, that is: (i) linear GRGDS, (ii) cyclo[RGDfK], and (iii) the knottin-RGD itself for binding to three different integrin receptors (αvβ3, αvβ5, α5β1). Major benefits of the novel assay are (i) the extremely low consumption of integrin (50 ng/peptide), (ii) the fact that neither antibodies/ECM-proteins nor integrin-expressing cells are required for detection, and (iii) its suitability for high-throughput screening of (RGD-)peptide libraries.
中文翻译:

高亲和力RGD-打结素肽作为新工具未标记的RGD肽的α到粘结强度的快速评价v β 3,α v β 5,α 5 β 1个整联蛋白受体
我们描述了一种高度敏感的竞争ELISA,无需使用细胞,ECM蛋白或抗体即可测量高通量的RGD肽的整联蛋白结合。该测定法测量(未标记的)RGD肽抑制生物素化的“结蛋白” -RGD肽与表面固定的整联蛋白结合的能力,因此可以定量高,中和低亲和力RGD的结合强度-黏合剂。我们介绍了生物素化的结蛋白-RGD肽,而不是生物素化的环[RGDfK](如Piras等人所报道),因为对于所有三种整联蛋白而言,整联蛋白的结合都更强并且可以清楚地检测到。为了最大程度地提高灵敏度和成本效益,我们首先优化了几个参数,例如整联蛋白固定化水平,结蛋白-RGD浓度,缓冲液组成,检测标签的类型(生物素,His或cMyc标签)和间隔物长度。因此,我们确定了两个关键因素,即(i)关键间隔区长度(比Gly长)和(ii)在所有孵育和洗涤缓冲液中均存在Ca 2+和Mg 2+。打结素-RGD肽的结合最强对于α v β 3,但也可检测到两个α v β 5和α 5 β 1,生物素化的同时,结合环[RGDfK]非常弱并且仅用于检测α v β 3。为了进行分析验证,我们最终确定了三种未标记肽的IC 50值,即:(i)线性GRGDS,(ii)环[RGDfK]和(iii)结蛋白-RGD自身与三种不同整合素受体(α v β 3,α v β 5,α 5 β 1)。在新的分析主要好处是(我)整合素的消耗极低(50 ng /肽),(ii)检测不需要抗体/ ECM蛋白或表达整合素的细胞,以及(iii)适用于(RGD)高通量筛选的事实-)肽库。
更新日期:2017-05-25
中文翻译:

高亲和力RGD-打结素肽作为新工具未标记的RGD肽的α到粘结强度的快速评价v β 3,α v β 5,α 5 β 1个整联蛋白受体
我们描述了一种高度敏感的竞争ELISA,无需使用细胞,ECM蛋白或抗体即可测量高通量的RGD肽的整联蛋白结合。该测定法测量(未标记的)RGD肽抑制生物素化的“结蛋白” -RGD肽与表面固定的整联蛋白结合的能力,因此可以定量高,中和低亲和力RGD的结合强度-黏合剂。我们介绍了生物素化的结蛋白-RGD肽,而不是生物素化的环[RGDfK](如Piras等人所报道),因为对于所有三种整联蛋白而言,整联蛋白的结合都更强并且可以清楚地检测到。为了最大程度地提高灵敏度和成本效益,我们首先优化了几个参数,例如整联蛋白固定化水平,结蛋白-RGD浓度,缓冲液组成,检测标签的类型(生物素,His或cMyc标签)和间隔物长度。因此,我们确定了两个关键因素,即(i)关键间隔区长度(比Gly长)和(ii)在所有孵育和洗涤缓冲液中均存在Ca 2+和Mg 2+。打结素-RGD肽的结合最强对于α v β 3,但也可检测到两个α v β 5和α 5 β 1,生物素化的同时,结合环[RGDfK]非常弱并且仅用于检测α v β 3。为了进行分析验证,我们最终确定了三种未标记肽的IC 50值,即:(i)线性GRGDS,(ii)环[RGDfK]和(iii)结蛋白-RGD自身与三种不同整合素受体(α v β 3,α v β 5,α 5 β 1)。在新的分析主要好处是(我)整合素的消耗极低(50 ng /肽),(ii)检测不需要抗体/ ECM蛋白或表达整合素的细胞,以及(iii)适用于(RGD)高通量筛选的事实-)肽库。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号