当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Monofluoromethyl‐Substituted Sulfonium Ylides: Electrophilic Monofluoromethylating Reagents with Broad Substrate Scopes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-10 , DOI: 10.1002/anie.201704175 Yafei Liu 1 , Long Lu 1 , Qilong Shen 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-10 , DOI: 10.1002/anie.201704175 Yafei Liu 1 , Long Lu 1 , Qilong Shen 1
Affiliation
|
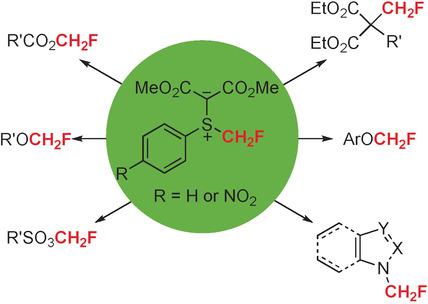
Two electrophilic monofluoromethylating reagents, monofluoromethyl(phenyl)sulfonium bis(carbomethoxy)methylide (3 a) and monofluoromethyl(4‐nitrophenyl)sulfonium bis(carbomethoxy)methylide (3 b), and their reactions under mild conditions with a variety of nucleophiles, such as alcohols and malonate derivatives, sulfonic and carboxylic acids, phenols, amides, and N heteroarenes, are described. Mechanistic studies with deuterated reagents [D2]3 a/[D2]3 b suggest that these monofluoromethylation reactions proceed through an electrophilic substitution pathway.
中文翻译:

单氟甲基取代的Y盐:具有广泛底物范围的亲电单氟甲基化试剂
两种亲电单氟甲基化试剂,单氟甲基(苯基)ulf双(羰甲氧基)甲基化物(3a)和单氟甲基(4-硝基苯基)ulf双(羰甲氧基)甲基化物(3b),以及它们在温和条件下与各种亲核试剂的反应作为醇和丙二酸酯的衍生物,描述了磺酸和羧酸,酚,酰胺和N杂芳烃。用氘化试剂[D 2 ] 3 a / [D 2 ] 3 b进行的机理研究表明,这些单氟甲基化反应是通过亲电取代途径进行的。
更新日期:2017-07-10
中文翻译:

单氟甲基取代的Y盐:具有广泛底物范围的亲电单氟甲基化试剂
两种亲电单氟甲基化试剂,单氟甲基(苯基)ulf双(羰甲氧基)甲基化物(3a)和单氟甲基(4-硝基苯基)ulf双(羰甲氧基)甲基化物(3b),以及它们在温和条件下与各种亲核试剂的反应作为醇和丙二酸酯的衍生物,描述了磺酸和羧酸,酚,酰胺和N杂芳烃。用氘化试剂[D 2 ] 3 a / [D 2 ] 3 b进行的机理研究表明,这些单氟甲基化反应是通过亲电取代途径进行的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号