当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of Thiol–yne/Thiol–ene Reactions for Peptide and Protein Macrocyclizations
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2017-04-26 06:43:04 , DOI: 10.1002/chem.201700572 Yuanxiang Wang 1 , Benjamin J. Bruno 2 , Sean Cornillie 3 , Jason M. Nogieira 1 , Diao Chen 1 , Thomas E. Cheatham 3 , Carol S. Lim 2 , Danny Hung-Chieh Chou 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2017-04-26 06:43:04 , DOI: 10.1002/chem.201700572 Yuanxiang Wang 1 , Benjamin J. Bruno 2 , Sean Cornillie 3 , Jason M. Nogieira 1 , Diao Chen 1 , Thomas E. Cheatham 3 , Carol S. Lim 2 , Danny Hung-Chieh Chou 1
Affiliation
|
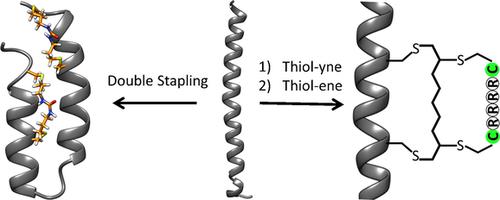
The application of thiol–yne/thiol–ene reactions to synthesize mono- and bicyclic-stapled peptides and proteins is reported. First, a thiol–ene-based peptide-stapling method in aqueous conditions was developed. This method enabled the efficient stapling of recombinantly expressed coil-coiled proteins. The resulting stapled protein demonstrated higher stability in its secondary structure than the unstapled version. Furthermore, a thiol–yne coupling was performed by using an α,ω-diyne to react with two cysteine residues to synthesize a stapled peptide with two vinyl sulfide groups. The stapled peptide could further react with another biscysteine peptide to yield a bicyclic stapled peptide with enhanced properties. For example, the cell permeability of a stapled peptide was further increased by appending an oligoarginine cell-penetrating peptide. The robustness and versatility of thiol–yne/thiol–ene reactions that can be applied to both synthetic and expressed peptides and proteins were demonstrated.
中文翻译:

硫醇-炔/硫醇-烯反应在肽和蛋白质大环化中的应用
据报道,硫醇-炔/硫醇-烯反应在合成单环和双环装订的肽和蛋白质中的应用。首先,开发了在水性条件下基于硫醇烯的肽装订方法。该方法使得能够有效地装订重组表达的卷曲螺旋蛋白。所得的钉合蛋白在二级结构中显示出比未钉合蛋白更高的稳定性。此外,通过使用α,ω-二炔与两个半胱氨酸残基反应,以合成具有两个乙烯基硫醚基团的固定肽,进行了硫醇-炔偶联。所述钉合肽可以进一步与另一双半胱氨酸肽反应以产生具有增强性质的双环钉合肽。例如,通过附加寡精氨酸穿透细胞的肽,进一步增加了吻合肽的细胞通透性。
更新日期:2017-05-24
中文翻译:

硫醇-炔/硫醇-烯反应在肽和蛋白质大环化中的应用
据报道,硫醇-炔/硫醇-烯反应在合成单环和双环装订的肽和蛋白质中的应用。首先,开发了在水性条件下基于硫醇烯的肽装订方法。该方法使得能够有效地装订重组表达的卷曲螺旋蛋白。所得的钉合蛋白在二级结构中显示出比未钉合蛋白更高的稳定性。此外,通过使用α,ω-二炔与两个半胱氨酸残基反应,以合成具有两个乙烯基硫醚基团的固定肽,进行了硫醇-炔偶联。所述钉合肽可以进一步与另一双半胱氨酸肽反应以产生具有增强性质的双环钉合肽。例如,通过附加寡精氨酸穿透细胞的肽,进一步增加了吻合肽的细胞通透性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号