当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Modification of a Dehydratase Enzyme Involved in Bacterial Virulence by an Ammonium Derivative: Evidence of its Active Site Covalent Adduct
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-07-16 , DOI: 10.1021/jacs.5b04080 Concepción González-Bello , Lorena Tizón , Emilio Lence , José M. Otero , Mark J. van Raaij 1 , Marta Martinez-Guitian 2 , Alejandro Beceiro 2 , Paul Thompson 3 , Alastair R. Hawkins 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-07-16 , DOI: 10.1021/jacs.5b04080 Concepción González-Bello , Lorena Tizón , Emilio Lence , José M. Otero , Mark J. van Raaij 1 , Marta Martinez-Guitian 2 , Alejandro Beceiro 2 , Paul Thompson 3 , Alastair R. Hawkins 3
Affiliation
|
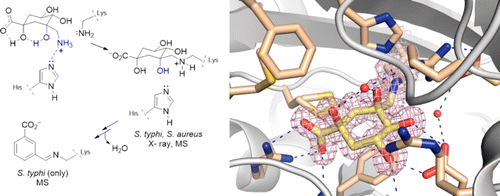
The first example of an ammonium derivative that causes a specific modification of the active site of type I dehydroquinase (DHQ1), a dehydratase enzyme that is a promising target for antivirulence drug discovery, is described. The resolution at 1.35 Å of the crystal structure of DHQ1 from Salmonella typhi chemically modified by this ammonium derivative revealed that the ligand is covalently attached to the essential Lys170 through the formation of an amine. The detection by mass spectroscopy of the reaction intermediates, in conjunction with the results of molecular dynamics simulations, allowed us to explain the inhibition mechanism and the experimentally observed differences between S. typhi and Staphylococcus aureus enzymes. The results presented here reveal that the replacement of Phe225 in St-DHQ1 by Tyr214 in Sa-DHQ1 and its hydrogen bonding interaction with the conserved water molecule observed in several crystal structures protects the amino adduct against further dehydration/aromatization reactions. In contrast, for the St-DHQ1 enzyme, the carboxylate group of Asp114, with the assistance of this water molecule, would trigger the formation of a Schiff base that can undergo further dehydration reactions until full aromatization of the cyclohexane ring is achieved. Moreover, in vitro antivirulence studies showed that the reported compound is able to reduce the ability of Salmonella Enteritidis to kill A459 respiratory cells. These studies have identified a good scaffold for the design of irreversible inhibitors that can be used as drugs and has opened up new opportunities for the development of novel antivirulence agents by targeting the DHQ1 enzyme.
中文翻译:

铵衍生物对参与细菌毒力的脱水酶的化学修饰:其活性位点共价加合物的证据
描述了引起 I 型脱氢奎宁酶 (DHQ1) 活性位点特定修饰的铵衍生物的第一个例子,该酶是一种有希望的抗毒药物发现靶点。伤寒沙门氏菌的 DHQ1 晶体结构分辨率为 1.35 Å,由该铵衍生物化学修饰,表明该配体通过形成胺共价连接到必需的 Lys170。反应中间体的质谱检测,结合分子动力学模拟的结果,使我们能够解释抑制机制以及伤寒链球菌和金黄色葡萄球菌酶之间的实验观察差异。此处呈现的结果表明,Sa-DHQ1 中的 Tyr214 替换 St-DHQ1 中的 Phe225 及其与在几种晶体结构中观察到的保守水分子的氢键相互作用保护氨基加合物免受进一步的脱水/芳香化反应。相比之下,对于 St-DHQ1 酶,Asp114 的羧酸根基团在该水分子的帮助下,会触发席夫碱的形成,席夫碱可以进行进一步的脱水反应,直到实现环己烷环的完全芳构化。此外,体外抗毒力研究表明,报道的化合物能够降低肠炎沙门氏菌杀死 A459 呼吸道细胞的能力。
更新日期:2015-07-16
中文翻译:

铵衍生物对参与细菌毒力的脱水酶的化学修饰:其活性位点共价加合物的证据
描述了引起 I 型脱氢奎宁酶 (DHQ1) 活性位点特定修饰的铵衍生物的第一个例子,该酶是一种有希望的抗毒药物发现靶点。伤寒沙门氏菌的 DHQ1 晶体结构分辨率为 1.35 Å,由该铵衍生物化学修饰,表明该配体通过形成胺共价连接到必需的 Lys170。反应中间体的质谱检测,结合分子动力学模拟的结果,使我们能够解释抑制机制以及伤寒链球菌和金黄色葡萄球菌酶之间的实验观察差异。此处呈现的结果表明,Sa-DHQ1 中的 Tyr214 替换 St-DHQ1 中的 Phe225 及其与在几种晶体结构中观察到的保守水分子的氢键相互作用保护氨基加合物免受进一步的脱水/芳香化反应。相比之下,对于 St-DHQ1 酶,Asp114 的羧酸根基团在该水分子的帮助下,会触发席夫碱的形成,席夫碱可以进行进一步的脱水反应,直到实现环己烷环的完全芳构化。此外,体外抗毒力研究表明,报道的化合物能够降低肠炎沙门氏菌杀死 A459 呼吸道细胞的能力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号