Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-05-19 , DOI: 10.1016/j.bmc.2017.05.041 Dylan T. Marsh , Sukanya Das , Jessica Ridell , Scott D. Smid
|
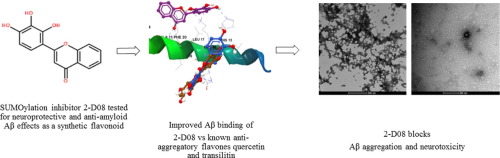
Naturally-occurring flavonoids have well documented anti-aggregatory and neuroprotective properties against the hallmark toxic protein in Alzheimer’s disease, amyloid β (Aβ). However the extensive diversity of flavonoids has limited the insight into the precise structure-activity relationships that confer such bioactive properties against the Aβ protein. In the present study we have characterised the Aβ binding properties, anti-aggregatory and neuroprotective effects of a discreet set of flavones, including the recently described novel protein sumoylation inhibitor 2′,3′,4′-trihydroxyflavone (2-D08). Quercetin, transilitin, jaceosidin, nobiletin and 2-D08 were incubated with human Aβ1–42 for 48 h in vitro and effects on Aβ fibrillisation kinetics and morphology measured using Thioflavin T (ThT) and electron microscopy respectively, in addition to effects on neuronal PC12 cell viability. Of the flavones studied, only quercetin, transilitin and 2-D08 significantly inhibited Aβ1–42 aggregation and toxicity in PC12 cells. Of those, 2-D08 was the most effective inhibitor. The strong anti-amyloid activity of 2-D08 indicates that extensive hydroxylation in the B ring is the most important determinant of activity against β amyloid within the flavone scaffold. The lack of efficacy of jaceosidin and nobiletin indicate that extension of B ring hydroxylation with methoxyl groups result in an incremental loss of anti-fibrillar and neuroprotective activity, highlighting the constraint to vicinal hydroxyl groups in the B ring for effective inhibition of aggregation. These findings reveal further structural insights into anti-amyloid bioactivity of flavonoids in addition to a novel and efficacious anti-aggregatory and neuroprotective effect of the semi-synthetic flavone and sumoylation inhibitor 2′,3′,4′-trihydroxyflavone (2-D08). Such modified flavones may facilitate drug development targeting multiple pathways in neurodegenerative disease.
中文翻译:

黄酮与淀粉样蛋白β相互作用的结构活性关系揭示了2',3',4'-三羟基黄酮(2-D08)的新型抗聚集和神经保护作用
天然存在的类黄酮具有针对阿尔茨海默氏病(淀粉样蛋白β(Aβ))中标志性毒性蛋白的抗聚集和神经保护特性。然而,类黄酮的广泛多样性限制了对精确的结构-活性关系的认识,这些关系赋予了这种抗Aβ蛋白生物活性的特性。在本研究中,我们已经表征了一组谨慎的黄酮的Aβ结合特性,抗聚集和神经保护作用,包括最近描述的新型蛋白质磺酰化抑制剂2',3',4'-三羟基黄酮(2-D08)。将槲皮素,transilitin,jaceosidin,nobiletin和2-D08与人Aβ1–42一起在体外孵育48小时除了对神经元PC12细胞活力的影响外,还分别使用硫黄素T(ThT)和电子显微镜对Aβ纤维化动力学和形态进行了测定。在所研究的黄酮中,只有槲皮素,transilitin和2-D08显着抑制Aβ1–42在PC12细胞中的聚集和毒性。其中,2-D08是最有效的抑制剂。2-D08的强抗淀粉样蛋白活性表明B环中广泛的羟基化作用是黄酮支架中针对β淀粉样蛋白的活性最重要的决定因素。缺乏鞘氨醇和诺比列汀的功效表明,用甲氧基基团扩大B环羟基化会导致抗原纤维和神经保护活性的损失逐渐增加,从而突显了对B环中邻位羟基的限制,以有效抑制聚集。这些发现揭示了对黄酮类化合物的抗淀粉样蛋白生物活性的进一步结构见解,以及半合成黄酮和磺酰化抑制剂2',3',4'-三羟基黄酮(2-D08)的新颖有效的抗聚集和神经保护作用。 。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号