当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bio-Based Functional Styrene Monomers Derived from Naturally Occurring Ferulic Acid for Poly(vinylcatechol) and Poly(vinylguaiacol) via Controlled Radical Polymerization
Macromolecules ( IF 5.1 ) Pub Date : 2017-05-19 00:00:00 , DOI: 10.1021/acs.macromol.7b00970 Hisaaki Takeshima 1 , Kotaro Satoh 1, 2 , Masami Kamigaito 1
Macromolecules ( IF 5.1 ) Pub Date : 2017-05-19 00:00:00 , DOI: 10.1021/acs.macromol.7b00970 Hisaaki Takeshima 1 , Kotaro Satoh 1, 2 , Masami Kamigaito 1
Affiliation
|
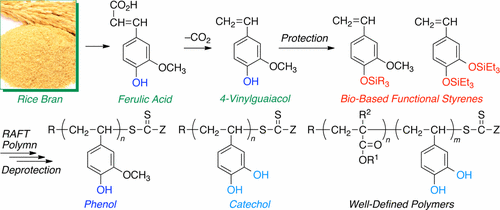
A naturally occurring vinylphenolic compound, 4-vinylguaiacol (4VG: 4-hydroxy-3-methoxystyrene), which is derived from naturally occurring ferulic acid via decarboxylation, was used for the synthesis of well-defined bio-based poly(vinylguaiacol) and poly(vinylcatechol) with phenolic functions. Through one-step chemical conversions of 4VG, a series of 4VG derivatives protected with acetyl (Ac4VG), tert-butyldimethylsilyl (TBDMS4VG), or triethylsilyl (TBDMS4VG) groups as well as bis(triethylsilyl)-protected vinyl catechol (TES2VC) were synthesized with high yields (>90%). The controlled radical polymerization of these protected bio-based phenolic styrene monomers successfully proceeded in the presence of appropriate reversible addition–fragmentation chain transfer (RAFT) agents or alkoxyamines with or without radical initiators such as 4,4′-azobis(isobutyronitrile) (AIBN) to result in polymers with controlled molecular weights. Bimolecular combination reactions were suppressed even at the high monomer conversions of >95%, especially for TES2VC possessing two bulky substituents, indicating the excellent living character of the polymerization in comparison to other styrene monomers. Deprotection of the silyl groups was easily attained with hydrochloric acid in THF and water to result in well-defined poly(vinylguaiacol) and poly(vinylcatechol) without loss of the RAFT terminals. These bio-based polystyrenes with phenolic functions were soluble in methanol and alkaline solution. The block copolymerization of TES2VC was accomplished with various common vinyl monomers, such as styrene, methyl methacrylate, methyl acrylate, and n-butyl acrylate, in the presence of their prepolymers as macro-RAFT agents, resulting in well-defined catechol-containing block copolymers after deprotection without any damage to the ester substituents and RAFT terminals.
中文翻译:

天然存在的阿魏酸衍生的基于生物的功能性苯乙烯单体,通过受控的自由基聚合反应生成聚(乙烯基邻苯二酚)和聚(乙烯基愈创木酚)
天然存在的乙烯基酚化合物4-乙烯基愈创木酚(4VG:4-羟基-3-甲氧基苯乙烯)是通过脱羧作用从天然阿魏酸衍生而来的,用于合成定义明确的生物基聚乙烯基愈创木酚和(乙烯基邻苯二酚)具有酚功能。通过4VG,一系列与乙酰基(Ac4VG)保护4VG衍生物,一步法化学转化叔丁基二(TBDMS4VG),或三乙基(TBDMS4VG)基团,以及二(三乙基甲硅烷)保护的乙烯基儿茶酚(TES 2VC)以高收率(> 90%)合成。在有或没有自由基引发剂(例如4,4'-偶氮二异丁腈)(AIBN)的适当可逆加成-断裂链转移(RAFT)试剂或烷氧基胺的存在下,这些受保护的生物基酚醛苯乙烯单体的受控自由基聚合成功进行。 ),以制得分子量可控的聚合物。即使在> 95%的高单体转化率下,双分子组合反应也被抑制,尤其是对于TES 2VC具有两个庞大的取代基,与其他苯乙烯单体相比,表明聚合反应具有出色的活性。用盐酸在THF和水中的去甲硅烷基的脱保护很容易实现,从而得到定义明确的聚(乙烯基愈创木酚)和聚(乙烯基邻苯二酚),而没有失去RAFT末端。这些具有酚功能的生物基聚苯乙烯可溶于甲醇和碱性溶液。TES 2 VC的嵌段共聚反应是通过各种常见的乙烯基单体完成的,例如苯乙烯,甲基丙烯酸甲酯,丙烯酸甲酯和n丙烯酸丁酯在其预聚物作为大分子RAFT剂存在下,脱保护后可得到定义明确的含邻苯二酚的嵌段共聚物,而不会损坏酯取代基和RAFT末端。
更新日期:2017-05-19
中文翻译:

天然存在的阿魏酸衍生的基于生物的功能性苯乙烯单体,通过受控的自由基聚合反应生成聚(乙烯基邻苯二酚)和聚(乙烯基愈创木酚)
天然存在的乙烯基酚化合物4-乙烯基愈创木酚(4VG:4-羟基-3-甲氧基苯乙烯)是通过脱羧作用从天然阿魏酸衍生而来的,用于合成定义明确的生物基聚乙烯基愈创木酚和(乙烯基邻苯二酚)具有酚功能。通过4VG,一系列与乙酰基(Ac4VG)保护4VG衍生物,一步法化学转化叔丁基二(TBDMS4VG),或三乙基(TBDMS4VG)基团,以及二(三乙基甲硅烷)保护的乙烯基儿茶酚(TES 2VC)以高收率(> 90%)合成。在有或没有自由基引发剂(例如4,4'-偶氮二异丁腈)(AIBN)的适当可逆加成-断裂链转移(RAFT)试剂或烷氧基胺的存在下,这些受保护的生物基酚醛苯乙烯单体的受控自由基聚合成功进行。 ),以制得分子量可控的聚合物。即使在> 95%的高单体转化率下,双分子组合反应也被抑制,尤其是对于TES 2VC具有两个庞大的取代基,与其他苯乙烯单体相比,表明聚合反应具有出色的活性。用盐酸在THF和水中的去甲硅烷基的脱保护很容易实现,从而得到定义明确的聚(乙烯基愈创木酚)和聚(乙烯基邻苯二酚),而没有失去RAFT末端。这些具有酚功能的生物基聚苯乙烯可溶于甲醇和碱性溶液。TES 2 VC的嵌段共聚反应是通过各种常见的乙烯基单体完成的,例如苯乙烯,甲基丙烯酸甲酯,丙烯酸甲酯和n丙烯酸丁酯在其预聚物作为大分子RAFT剂存在下,脱保护后可得到定义明确的含邻苯二酚的嵌段共聚物,而不会损坏酯取代基和RAFT末端。































 京公网安备 11010802027423号
京公网安备 11010802027423号