当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulation of Antimalarial Activity at a Putative Bisquinoline Receptor In Vivo Using Fluorinated Bisquinolines
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2017-04-21 03:26:01 , DOI: 10.1002/chem.201605099
Alistair J. Fielding 1 , Valentina Lukinović 1 , Philip G. Evans 2 , Said Alizadeh-Shekalgourabi 3 , Roger H. Bisby 4 , Michael G. B. Drew 5 , Verity Male 5 , Alessio Del Casino 6 , James F. Dunn 6 , Laura E. Randle 6 , Nicola M. Dempster 6 , Lutfun Nahar 6 , Satyajit D. Sarker 6 , Fabián G. Cantú Reinhard 7 , Sam P. de Visser 7 , Mike J. Dascombe 8 , Fyaz M. D. Ismail 6
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2017-04-21 03:26:01 , DOI: 10.1002/chem.201605099
Alistair J. Fielding 1 , Valentina Lukinović 1 , Philip G. Evans 2 , Said Alizadeh-Shekalgourabi 3 , Roger H. Bisby 4 , Michael G. B. Drew 5 , Verity Male 5 , Alessio Del Casino 6 , James F. Dunn 6 , Laura E. Randle 6 , Nicola M. Dempster 6 , Lutfun Nahar 6 , Satyajit D. Sarker 6 , Fabián G. Cantú Reinhard 7 , Sam P. de Visser 7 , Mike J. Dascombe 8 , Fyaz M. D. Ismail 6
Affiliation
|
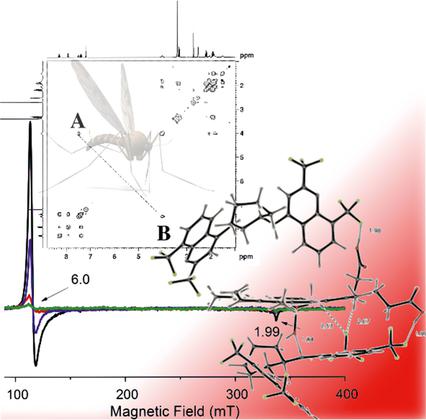
Antimalarials can interact with heme covalently, by π⋅⋅⋅π interactions or by hydrogen bonding. Consequently, the prototropy of 4-aminoquinolines and quinoline methanols was investigated by using quantum mechanics. Calculations showed mefloquine protonated preferentially at the piperidine and was impeded at the endocyclic nitrogen because of electronic rather than steric factors. In gas-phase calculations, 7-substituted mono- and bis-4-aminoquinolines were preferentially protonated at the endocyclic quinoline nitrogen. By contrast, compounds with a trifluoromethyl substituent on both the 2- and 8-positions, reversed the order of protonation, which now favored the exocyclic secondary amine nitrogen at the 4-position. Loss of antimalarial efficacy by CF3 groups simultaneously occupying the 2- and 8-positions was recovered if the CF3 group occupied the 7-position. Hence, trifluoromethyl groups buttressing the quinolinyl nitrogen shifted binding of antimalarials to hematin, enabling switching from endocyclic to the exocyclic N. Both theoretical calculations (DFT calculations: B3LYP/BS1) and crystal structure of (±)-trans-N1,N2-bis-(2,8-ditrifluoromethylquinolin-4-yl)cyclohexane-1,2-diamine were used to reveal the preferred mode(s) of interaction with hematin. The order of antimalarial activity in vivo followed the capacity for a redox change of the iron(III) state, which has important implications for the future rational design of 4-aminoquinoline antimalarials.
中文翻译:

使用氟化的双喹啉在假定的双喹啉受体体内抗疟活性的调节。
抗疟药可以通过π⋅⋅⋅π相互作用或氢键与血红素共价相互作用。因此,利用量子力学研究了4-氨基喹啉和喹啉甲醇的原生质。计算表明,甲氟喹优先在哌啶质子化,由于电子因素而非空间因素,其在环内氮原子处受阻。在气相计算中,在环内喹啉氮上优先质子交换了7-取代的单和双-4-氨基喹啉。相比之下,在2位和8位均具有三氟甲基取代基的化合物则颠倒了质子化的顺序,这现在有利于在4位上的环外仲胺氮。CF 3失去抗疟功效如果CF 3组占据7位,则恢复同时占据2和8位的基团。因此,支持喹啉基氮的三氟甲基基团使抗疟疾药物与血红素的结合发生了转移,从而从环内N转换为环外N。理论计算(DFT计算:B3LYP / BS1)和(±)-反式N 1,N 2的晶体结构使用-双-(2,8-二三氟甲基喹啉-4-基)环己烷-1,2-二胺来揭示与血红素相互作用的优选方式。体内抗疟活性的顺序遵循铁(III)状态的氧化还原变化的能力,这对未来4-氨基喹啉抗疟剂的合理设计具有重要意义。
更新日期:2017-05-19
中文翻译:

使用氟化的双喹啉在假定的双喹啉受体体内抗疟活性的调节。
抗疟药可以通过π⋅⋅⋅π相互作用或氢键与血红素共价相互作用。因此,利用量子力学研究了4-氨基喹啉和喹啉甲醇的原生质。计算表明,甲氟喹优先在哌啶质子化,由于电子因素而非空间因素,其在环内氮原子处受阻。在气相计算中,在环内喹啉氮上优先质子交换了7-取代的单和双-4-氨基喹啉。相比之下,在2位和8位均具有三氟甲基取代基的化合物则颠倒了质子化的顺序,这现在有利于在4位上的环外仲胺氮。CF 3失去抗疟功效如果CF 3组占据7位,则恢复同时占据2和8位的基团。因此,支持喹啉基氮的三氟甲基基团使抗疟疾药物与血红素的结合发生了转移,从而从环内N转换为环外N。理论计算(DFT计算:B3LYP / BS1)和(±)-反式N 1,N 2的晶体结构使用-双-(2,8-二三氟甲基喹啉-4-基)环己烷-1,2-二胺来揭示与血红素相互作用的优选方式。体内抗疟活性的顺序遵循铁(III)状态的氧化还原变化的能力,这对未来4-氨基喹啉抗疟剂的合理设计具有重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号