当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyroglutamate-modified Aβ(3-42) affects aggregation kinetics of Aβ(1-42) by accelerating primary and secondary pathways
Chemical Science ( IF 7.6 ) Pub Date : 2017-05-05 00:00:00 , DOI: 10.1039/c6sc04797a C. Dammers 1, 2, 3, 4 , M. Schwarten 1, 2, 3, 4 , A. K. Buell 4, 5, 6, 7 , D. Willbold 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-05-05 00:00:00 , DOI: 10.1039/c6sc04797a C. Dammers 1, 2, 3, 4 , M. Schwarten 1, 2, 3, 4 , A. K. Buell 4, 5, 6, 7 , D. Willbold 1, 2, 3, 4, 5
Affiliation
|
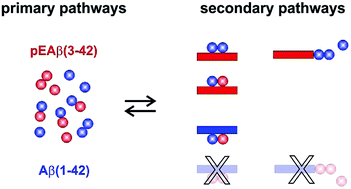
The aggregation into amyloid fibrils of amyloid-β (Aβ) peptides is a hallmark of Alzheimer's disease. A variety of Aβ peptides have been discovered in vivo, with pyroglutamate-modified Aβ (pEAβ) forming a significant proportion. pEAβ is mainly localized in the core of plaques, suggesting a possible role in inducing and facilitating Aβ oligomerization and accumulation. Despite this potential importance, the aggregation mechanism of pEAβ and its influence on the aggregation kinetics of other Aβ variants have not yet been elucidated. Here we show that pEAβ(3-42) forms fibrils much faster than Aβ(1-42) and the critical concentration above which aggregation was observed was drastically decreased by one order of magnitude compared to Aβ(1-42). We elucidated the co-aggregation mechanism of Aβ(1-42) with pEAβ(3-42). At concentrations at which both species do not aggregate as homofibrils, mixtures of pEAβ(3-42) and Aβ(1-42) aggregate, suggesting the formation of mixed nuclei. We show that the presence of pEAβ(3-42) monomers increases the rate of primary nucleation of Aβ(1-42) and that fibrils of pEAβ(3-42) serve as highly efficient templates for elongation and catalytic surfaces for secondary nucleation of Aβ(1-42). On the other hand, the addition of Aβ(1-42) monomers drastically decelerates the primary and secondary nucleation of pEAβ(3-42) while not altering the pEAβ(3-42) elongation rate. In addition, even moderate concentrations of fibrillar Aβ(1-42) prevent pEAβ(3-42) aggregation, likely due to non-reactive binding of pEAβ(3-42) monomers to the surfaces of Aβ(1-42) fibrils. Thus, pEAβ(3-42) accelerates aggregation of Aβ(1-42) by affecting all individual reaction steps of the aggregation process while Aβ(1-42) dramatically slows down the primary and secondary nucleation of pEAβ(3-42).
中文翻译:

焦谷氨酸修饰的Aβ(3-42)通过加速主要和次要途径影响Aβ(1-42)的聚集动力学
淀粉样蛋白-β(Aβ)肽聚集到淀粉样蛋白原纤维中是阿尔茨海默氏病的标志。体内发现了多种Aβ肽,其中焦谷氨酸修饰的Aβ(pEAβ)占很大比例。pEAβ主要位于斑块的核心,提示可能在诱导和促进Aβ寡聚和积累中发挥作用。尽管具有潜在的重要性,但尚未阐明pEAβ的聚集机理及其对其他Aβ变体的聚集动力学的影响。在这里,我们显示pEAβ(3-42)形成原纤维的速度比Aβ(1-42)快得多,与Aβ(1-42)相比,观察到该浓度的临界浓度急剧降低了一个数量级。我们阐明了Aβ(1-42)与pEAβ(3-42)的共聚集机制。在两种物质均不聚集为均原纤维的浓度下,pEAβ(3-42)和Aβ(1-42)的混合物聚集,表明形成了混合核。我们显示pEAβ(3-42)单体的存在增加了Aβ(1-42)的初级成核速率,并且pEAβ(3-42)的原纤维可作为高效的模板用于延伸和催化表面的二次成核Aβ(1-42)。另一方面,添加Aβ(1-42)单体可大大降低pEAβ(3-42)的初级和次级成核,而不会改变pEAβ(3-42)的延伸率。此外,即使中等浓度的原纤维Aβ(1-42)也会阻止pEAβ(3-42)聚集,这可能是由于pEAβ(3-42)单体与Aβ(1-42)原纤维表面的非反应性结合。因此,pEAβ(3-42)通过影响聚集过程的所有单个反应步骤来加速Aβ(1-42)的聚集,而Aβ(1-42)大大减慢了pEAβ(3-42)的初级和次级成核。
更新日期:2017-05-17
中文翻译:

焦谷氨酸修饰的Aβ(3-42)通过加速主要和次要途径影响Aβ(1-42)的聚集动力学
淀粉样蛋白-β(Aβ)肽聚集到淀粉样蛋白原纤维中是阿尔茨海默氏病的标志。体内发现了多种Aβ肽,其中焦谷氨酸修饰的Aβ(pEAβ)占很大比例。pEAβ主要位于斑块的核心,提示可能在诱导和促进Aβ寡聚和积累中发挥作用。尽管具有潜在的重要性,但尚未阐明pEAβ的聚集机理及其对其他Aβ变体的聚集动力学的影响。在这里,我们显示pEAβ(3-42)形成原纤维的速度比Aβ(1-42)快得多,与Aβ(1-42)相比,观察到该浓度的临界浓度急剧降低了一个数量级。我们阐明了Aβ(1-42)与pEAβ(3-42)的共聚集机制。在两种物质均不聚集为均原纤维的浓度下,pEAβ(3-42)和Aβ(1-42)的混合物聚集,表明形成了混合核。我们显示pEAβ(3-42)单体的存在增加了Aβ(1-42)的初级成核速率,并且pEAβ(3-42)的原纤维可作为高效的模板用于延伸和催化表面的二次成核Aβ(1-42)。另一方面,添加Aβ(1-42)单体可大大降低pEAβ(3-42)的初级和次级成核,而不会改变pEAβ(3-42)的延伸率。此外,即使中等浓度的原纤维Aβ(1-42)也会阻止pEAβ(3-42)聚集,这可能是由于pEAβ(3-42)单体与Aβ(1-42)原纤维表面的非反应性结合。因此,pEAβ(3-42)通过影响聚集过程的所有单个反应步骤来加速Aβ(1-42)的聚集,而Aβ(1-42)大大减慢了pEAβ(3-42)的初级和次级成核。































 京公网安备 11010802027423号
京公网安备 11010802027423号