当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkoxyallene-Based Stereodivergent Syntheses of (−)-Hyacinthacine B4 and of Putative Hyacinthacine C5 Epimers: Proposal of Hyacinthacine C5 Structure
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-05-08 00:00:00 , DOI: 10.1021/acs.joc.7b00667
Tommaso Pecchioli 1, 2 , Francesca Cardona 1 , Hans-Ulrich Reissig 2 , Reinhold Zimmer 2 , Andrea Goti 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-05-08 00:00:00 , DOI: 10.1021/acs.joc.7b00667
Tommaso Pecchioli 1, 2 , Francesca Cardona 1 , Hans-Ulrich Reissig 2 , Reinhold Zimmer 2 , Andrea Goti 1
Affiliation
|
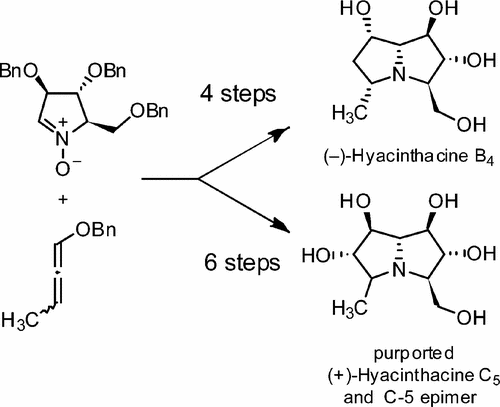
Hyacinthacines are members of the class of polyhydroxylated pyrrolizidines exhibiting outstanding biological activity as glycosidases inhibitors. Their structural complexity is embodied in the densely functionalized core, possessing a series of contiguous stereogenic centers. In this synthetic study we report a route to the more complex congeners of this class of alkaloids exploiting the diastereoselective addition of an axially chiral lithiated alkoxyallene to an enantiopure cyclic nitrone. Our stereodivergent approach enabled the installation of the targeted configuration at the ring A by minimal synthetic manipulations and at ring B by using stage dependent selective functionalizations. The versatility and robustness of this methodology were demonstrated by the syntheses of (−)-hyacinthacine B4 and of two epimers of (+)-hyacinthacine C5, allowing a suggestion of the likely structure of the isolated natural product.
中文翻译:

(-)-Hycinthacine B 4和假定的Hyacinthacine C 5差向异构体基于烷氧基丙二烯的立体发散合成:Hyacinthacine C 5结构的提议
扁豆氨酸是多羟基化的吡咯并核苷类的成员,其作为糖苷酶抑制剂表现出突出的生物学活性。它们的结构复杂性体现在功能密集的核心中,该核心具有一系列连续的立体生成中心。在这项综合研究中,我们报告了利用非手性将轴向手性锂化烷氧基丙二烯非对映选择性加成对映体纯的环状硝酮的途径,从而开发出此类生物碱的更复杂同类物。我们的立体发散方法可通过最少的合成操作将目标配置安装在A环上,并通过使用阶段依赖性的选择性功能化将其安装在B环上。该方法的多功能性和鲁棒性通过(-)-扁豆碱B 4的合成得到证明。和(+)-乙酰草氨酸C 5的两个差向异构体,暗示了分离的天然产物的可能结构。
更新日期:2017-05-17
中文翻译:

(-)-Hycinthacine B 4和假定的Hyacinthacine C 5差向异构体基于烷氧基丙二烯的立体发散合成:Hyacinthacine C 5结构的提议
扁豆氨酸是多羟基化的吡咯并核苷类的成员,其作为糖苷酶抑制剂表现出突出的生物学活性。它们的结构复杂性体现在功能密集的核心中,该核心具有一系列连续的立体生成中心。在这项综合研究中,我们报告了利用非手性将轴向手性锂化烷氧基丙二烯非对映选择性加成对映体纯的环状硝酮的途径,从而开发出此类生物碱的更复杂同类物。我们的立体发散方法可通过最少的合成操作将目标配置安装在A环上,并通过使用阶段依赖性的选择性功能化将其安装在B环上。该方法的多功能性和鲁棒性通过(-)-扁豆碱B 4的合成得到证明。和(+)-乙酰草氨酸C 5的两个差向异构体,暗示了分离的天然产物的可能结构。

































 京公网安备 11010802027423号
京公网安备 11010802027423号