当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting parameter space in MOFs: a 20-fold enhancement of phosphate-ester hydrolysis with UiO-66-NH2†
Chemical Science ( IF 7.6 ) Pub Date : 2015-02-24 00:00:00 , DOI: 10.1039/c4sc03613a Michael J Katz 1 , Su-Young Moon 1 , Joseph E Mondloch 1 , M Hassan Beyzavi 1 , Casey J Stephenson 1 , Joseph T Hupp 1, 2 , Omar K Farha 1, 3
Chemical Science ( IF 7.6 ) Pub Date : 2015-02-24 00:00:00 , DOI: 10.1039/c4sc03613a Michael J Katz 1 , Su-Young Moon 1 , Joseph E Mondloch 1 , M Hassan Beyzavi 1 , Casey J Stephenson 1 , Joseph T Hupp 1, 2 , Omar K Farha 1, 3
Affiliation
|
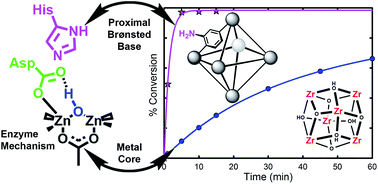
The hydrolysis of nerve agents is of primary concern due to the severe toxicity of these agents. Using a MOF-based catalyst (UiO-66), we have previously demonstrated that the hydrolysis can occur with relatively fast half-lives of 50 minutes. However, these rates are still prohibitively slow to be efficiently utilized for some practical applications (e.g., decontamination wipes used to clean exposed clothing/skin/vehicles). We thus turned our attention to derivatives of UiO-66 in order to probe the importance of functional groups on the hydrolysis rate. Three UiO-66 derivatives were explored; UiO-66-NO2 and UiO-66-(OH)2 showed little to no change in hydrolysis rate. However, UiO-66-NH2 showed a 20 fold increase in hydrolysis rate over the parent UiO-66 MOF. Half-lives of 1 minute were observed with this MOF. In order to probe the role of the amino moiety, we turned our attention to UiO-67, UiO-67-NMe2 and UiO-67-NH2. In these MOFs, the amino moiety is in close proximity to the zirconium node. We observed that UiO-67-NH2 is a faster catalyst than UiO-67 and UiO-67-NMe2. We conclude that the role of the amino moiety is to act as a proton-transfer agent during the catalytic cycle and not to hydrogen bond or to form a phosphorane intermediate.
中文翻译:

利用 MOF 中的参数空间:UiO-66-NH2 将磷酸酯水解增强 20 倍†
由于神经毒剂的严重毒性,它们的水解是首要关注的问题。使用基于 MOF 的催化剂 (UiO-66),我们之前已经证明水解可以以 50 分钟相对较快的半衰期发生。然而,这些速率对于有效地用于某些实际应用(例如,用于清洁暴露的衣服/皮肤/车辆的去污擦拭布)来说仍然非常慢。因此,我们将注意力转向 UiO-66 的衍生物,以探讨官能团对水解速率的重要性。探索了三种 UiO-66 衍生物;UiO-66-NO 2和UiO-66-(OH) 2 的水解速率几乎没有变化。然而,UiO-66-NH 2 的水解速率比母体UiO-66 MOF提高了20倍。使用该 MOF 观察到的半衰期为 1 分钟。为了探讨氨基部分的作用,我们将注意力转向UiO-67、UiO-67-NMe 2和UiO-67-NH 2。在这些 MOF 中,氨基部分非常靠近锆节点。我们观察到UiO-67-NH 2是比UiO-67 和UiO-67-NMe 2更快的催化剂。我们得出结论,氨基部分的作用是在催化循环过程中充当质子转移剂,而不是形成氢键或形成正膦中间体。
更新日期:2015-02-24
中文翻译:

利用 MOF 中的参数空间:UiO-66-NH2 将磷酸酯水解增强 20 倍†
由于神经毒剂的严重毒性,它们的水解是首要关注的问题。使用基于 MOF 的催化剂 (UiO-66),我们之前已经证明水解可以以 50 分钟相对较快的半衰期发生。然而,这些速率对于有效地用于某些实际应用(例如,用于清洁暴露的衣服/皮肤/车辆的去污擦拭布)来说仍然非常慢。因此,我们将注意力转向 UiO-66 的衍生物,以探讨官能团对水解速率的重要性。探索了三种 UiO-66 衍生物;UiO-66-NO 2和UiO-66-(OH) 2 的水解速率几乎没有变化。然而,UiO-66-NH 2 的水解速率比母体UiO-66 MOF提高了20倍。使用该 MOF 观察到的半衰期为 1 分钟。为了探讨氨基部分的作用,我们将注意力转向UiO-67、UiO-67-NMe 2和UiO-67-NH 2。在这些 MOF 中,氨基部分非常靠近锆节点。我们观察到UiO-67-NH 2是比UiO-67 和UiO-67-NMe 2更快的催化剂。我们得出结论,氨基部分的作用是在催化循环过程中充当质子转移剂,而不是形成氢键或形成正膦中间体。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号