当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boron Nitride: A Support for Highly Active Heteropolyacids in the Methanol-to-DME Reaction
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-05-04 00:00:00 , DOI: 10.1021/acscatal.7b00808 Josefine Schnee 1 , Adrien Eggermont 1 , Eric M. Gaigneaux 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-05-04 00:00:00 , DOI: 10.1021/acscatal.7b00808 Josefine Schnee 1 , Adrien Eggermont 1 , Eric M. Gaigneaux 1
Affiliation
|
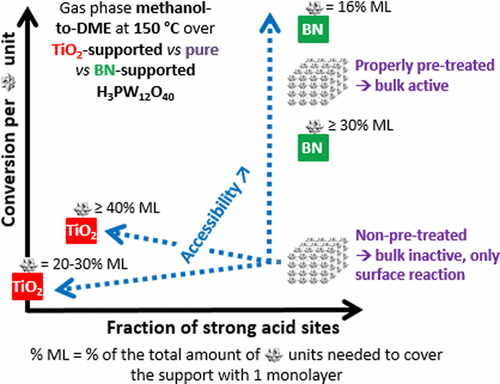
Due to its superacidity and ability of its bulk to react following a pseudoliquid mechanism, the Keggin H3PW12O40 heteropolyacid attracts more and more attention as a catalyst for the gas phase methanol-to-DME reaction. However, in its pure state, H3PW12O40 has a very low surface area (typically 5–10 m2/g), which limits the accessibility of its inner protons due to diffusional constraints and explains why teams investigate H3PW12O40 in its supported form. In the present work, we highlight the interest of using hexagonal boron nitride (BN) as a support. We show that, in contrast to commonly used supports such as TiO2, BN is able to increase the accessibility of H3PW12O40’s acid sites (i.e., stabilizing small enough crystallites) while preserving their strong acidity (i.e., not interacting too much with the Keggin units). At low loadings (typically around 16% of one ideal Keggin monolayer), BN leads H3PW12O40 to reach an almost 2 times higher methanol conversion than obtained with the adequately activated pure bulk sample, and an almost 10 times higher conversion than an optimized TiO2-supported H3PW12O40 catalyst. At higher H3PW12O40 loadings, the BN-supported catalysts are still much more active than the optimized TiO2-supported one, but less active than the pure bulk-activated H3PW12O40, which we attribute to the partial intercalation of H3PW12O40 within the interlayers of BN.
中文翻译:

氮化硼:甲醇制DME反应中高活性杂多酸的支持物
由于其超酸度和其本体遵循拟液机理反应的能力,Keggin H 3 PW 12 O 40杂多酸作为气相甲醇制DME反应的催化剂越来越受到关注。然而,在纯净状态下,H 3 PW 12 O 40的表面积非常低(通常为5-10 m 2 / g),由于扩散限制,它限制了其内部质子的可及性,并解释了为什么团队研究H 3 PW 12 Ô 40以其受支持的形式。在当前的工作中,我们强调了使用六方氮化硼(BN)作为载体的兴趣。我们表明,与常用的载体(如TiO 2)相比,BN能够增加H 3 PW 12 O 40的酸性位点的可及性(即稳定足够小的微晶),同时保留其强酸度(即不与Keggin单位互动过多)。在低负载下(通常约为一个理想的Keggin单层膜的16%),BN导致H 3 PW 12 O 40的甲醇转化率比充分活化的纯大块样品所获得的甲醇转化率高几乎2倍,而转化率则比其高出近10倍。优化的二氧化钛2负载的H 3 PW 12 O 40催化剂。在较高的H 3 PW 12 O 40负载下,BN负载的催化剂的活性仍然比优化的TiO 2负载的催化剂高得多,但比纯的本体活化的H 3 PW 12 O 40活性低,我们将其归因于H 3 PW 12 O 40在BN中间层中的部分嵌入。
更新日期:2017-05-13
中文翻译:

氮化硼:甲醇制DME反应中高活性杂多酸的支持物
由于其超酸度和其本体遵循拟液机理反应的能力,Keggin H 3 PW 12 O 40杂多酸作为气相甲醇制DME反应的催化剂越来越受到关注。然而,在纯净状态下,H 3 PW 12 O 40的表面积非常低(通常为5-10 m 2 / g),由于扩散限制,它限制了其内部质子的可及性,并解释了为什么团队研究H 3 PW 12 Ô 40以其受支持的形式。在当前的工作中,我们强调了使用六方氮化硼(BN)作为载体的兴趣。我们表明,与常用的载体(如TiO 2)相比,BN能够增加H 3 PW 12 O 40的酸性位点的可及性(即稳定足够小的微晶),同时保留其强酸度(即不与Keggin单位互动过多)。在低负载下(通常约为一个理想的Keggin单层膜的16%),BN导致H 3 PW 12 O 40的甲醇转化率比充分活化的纯大块样品所获得的甲醇转化率高几乎2倍,而转化率则比其高出近10倍。优化的二氧化钛2负载的H 3 PW 12 O 40催化剂。在较高的H 3 PW 12 O 40负载下,BN负载的催化剂的活性仍然比优化的TiO 2负载的催化剂高得多,但比纯的本体活化的H 3 PW 12 O 40活性低,我们将其归因于H 3 PW 12 O 40在BN中间层中的部分嵌入。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号