当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Aminocarbonylation in Solid-Phase Peptide Synthesis: A Method for Capping, Cyclization, and Isotope Labeling
Organic Letters ( IF 4.9 ) Pub Date : 2017-05-12 00:00:00 , DOI: 10.1021/acs.orglett.7b01068 Anna Skogh 1 , Stig D. Friis 2 , Troels Skrydstrup 2 , Anja Sandström 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-05-12 00:00:00 , DOI: 10.1021/acs.orglett.7b01068 Anna Skogh 1 , Stig D. Friis 2 , Troels Skrydstrup 2 , Anja Sandström 1
Affiliation
|
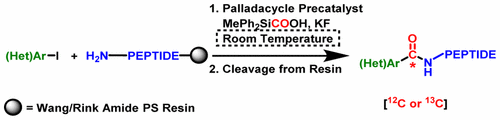
A new synthetic approach for introducing N-capping groups onto peptides attached to a solid support, combining aminocarbonylation under mild conditions using a palladacycle precatalyst and solid-phase peptide synthesis, is reported. The use of a silacarboxylic acid as an in situ CO-releasing molecule allowed the reaction to be performed in a single vial. The method also enables versatile substitution of side chains, side-chain-to-side-chain cyclizations, and selective [13C] acyl labeling of modified peptides.
中文翻译:

固相肽合成中钯催化的氨基羰基化:封端,环化和同位素标记的方法
报道了一种新的合成方法,该方法用于在结合有固相支持物的肽上引入N封端基团,并在适度条件下使用Palladacycle预催化剂和固相肽合成方法将氨基羰基化结合在一起。使用硅烷羧酸作为原位释放CO的分子使得反应可以在单个小瓶中进行。该方法还可以实现侧链的通用取代,侧链至侧链的环化以及修饰肽的选择性[ 13 C]酰基标记。
更新日期:2017-05-12
中文翻译:

固相肽合成中钯催化的氨基羰基化:封端,环化和同位素标记的方法
报道了一种新的合成方法,该方法用于在结合有固相支持物的肽上引入N封端基团,并在适度条件下使用Palladacycle预催化剂和固相肽合成方法将氨基羰基化结合在一起。使用硅烷羧酸作为原位释放CO的分子使得反应可以在单个小瓶中进行。该方法还可以实现侧链的通用取代,侧链至侧链的环化以及修饰肽的选择性[ 13 C]酰基标记。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号