当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coordinating Biointeraction and Bioreaction of a Nanocarrier Material and an Anticancer Drug to Overcome Membrane Rigidity and Target Mitochondria in Multidrug‐Resistant Cancer Cells
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-05-12 , DOI: 10.1002/adfm.201700804 Rui Xue Zhang 1 , Lily Yi Li 1 , Jason Li 1 , Zhensong Xu 2 , Azhar Z. Abbasi 1 , Lucy Lin 1 , Mohammad A. Amini 1 , Wei Yu Weng 3 , Yu Sun 2 , Andrew M. Rauth 4 , Xiao Yu Wu 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-05-12 , DOI: 10.1002/adfm.201700804 Rui Xue Zhang 1 , Lily Yi Li 1 , Jason Li 1 , Zhensong Xu 2 , Azhar Z. Abbasi 1 , Lucy Lin 1 , Mohammad A. Amini 1 , Wei Yu Weng 3 , Yu Sun 2 , Andrew M. Rauth 4 , Xiao Yu Wu 1
Affiliation
|
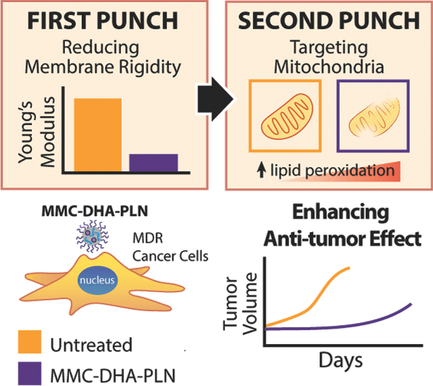
Multidrug resistance (MDR) is a main cause of chemotherapy failure in cancer treatment. It is associated with complex cellular and molecular mechanisms including overexpression of drug efflux transporters, increased membrane rigidity, and impaired apoptosis. Numerous efforts have been made to overcome efflux transporter‐mediated MDR using nanotechnology‐based approaches. However, these approaches fail to surmount plasma membrane rigidity that attenuates drug penetration and nanoparticle endocytosis. Here, a “one‐two punch” nanoparticle approach is proposed to coordinate intracellular biointeraction and bioreaction of a nanocarrier material docosahexaenoic acid (DHA) and an anticancer prodrug mitomycin C (MMC) to enhance mitochondrion‐targeted toxicity. Incorporation of DHA in solid polymer‐lipid nanoparticles first reduces the membrane rigidity in live cancer cells thereby increasing nanoparticle cellular uptake and MMC accumulation. Subsequent intracellular MMC bioreduction produces free radicals that in turn react with adjacent DHA inducing significantly elevated mitochondrial lipid peroxidation, leading to irreversible damage to mitochondria. Preferential tumor accumulation of the nanoparticles and the synergistic anticancer cytotoxicity remarkably inhibit tumor growth and prolonged host survival without any systemic toxicity in an orthotopic MDR breast tumor model. This work suggests that combinatorial use of biophysical and biochemical properties of nanocarrier materials with bioreactive prodrugs is a powerful approach to overcoming multifactorial MDR in cancer.
中文翻译:

协调纳米载体材料和抗癌药物的生物相互作用和生物反应,以克服多药耐药癌细胞中的膜刚性和靶线粒体
多药耐药性(MDR)是癌症治疗中化疗失败的主要原因。它与复杂的细胞和分子机制有关,包括药物外排转运蛋白的过表达,膜刚性增强和细胞凋亡受损。为了使用基于纳米技术的方法克服外排转运蛋白介导的MDR,已经做出了许多努力。但是,这些方法不能克服质膜的刚性,质膜的刚性会减弱药物的渗透和纳米颗粒的内吞作用。在这里,提出了一种“一两拳”的纳米粒子方法来协调细胞内生物相互作用和纳米载体材料二十二碳六烯酸(DHA)和抗癌前药丝裂霉素C(MMC)的生物反应,以增强针对线粒体的毒性。在固体聚合物脂质纳米颗粒中掺入DHA首先会降低活癌细胞的膜刚性,从而增加纳米颗粒细胞的摄取和MMC的积累。随后的细胞内MMC生物还原产生自由基,进而与邻近的DHA反应,诱导线粒体脂质过氧化显着升高,从而导致线粒体不可逆转的破坏。在原位MDR乳腺肿瘤模型中,纳米粒子的优先肿瘤蓄积和协同抗癌细胞毒性显着抑制了肿瘤的生长并延长了宿主的存活时间,而没有任何系统毒性。这项工作表明,纳米载体材料的生物物理和生化特性与生物反应性前药的组合使用是克服癌症中多因素MDR的有力方法。
更新日期:2017-05-12
中文翻译:

协调纳米载体材料和抗癌药物的生物相互作用和生物反应,以克服多药耐药癌细胞中的膜刚性和靶线粒体
多药耐药性(MDR)是癌症治疗中化疗失败的主要原因。它与复杂的细胞和分子机制有关,包括药物外排转运蛋白的过表达,膜刚性增强和细胞凋亡受损。为了使用基于纳米技术的方法克服外排转运蛋白介导的MDR,已经做出了许多努力。但是,这些方法不能克服质膜的刚性,质膜的刚性会减弱药物的渗透和纳米颗粒的内吞作用。在这里,提出了一种“一两拳”的纳米粒子方法来协调细胞内生物相互作用和纳米载体材料二十二碳六烯酸(DHA)和抗癌前药丝裂霉素C(MMC)的生物反应,以增强针对线粒体的毒性。在固体聚合物脂质纳米颗粒中掺入DHA首先会降低活癌细胞的膜刚性,从而增加纳米颗粒细胞的摄取和MMC的积累。随后的细胞内MMC生物还原产生自由基,进而与邻近的DHA反应,诱导线粒体脂质过氧化显着升高,从而导致线粒体不可逆转的破坏。在原位MDR乳腺肿瘤模型中,纳米粒子的优先肿瘤蓄积和协同抗癌细胞毒性显着抑制了肿瘤的生长并延长了宿主的存活时间,而没有任何系统毒性。这项工作表明,纳米载体材料的生物物理和生化特性与生物反应性前药的组合使用是克服癌症中多因素MDR的有力方法。






































 京公网安备 11010802027423号
京公网安备 11010802027423号