当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neutron and Atomic Resolution X-ray Structures of a Lytic Polysaccharide Monooxygenase Reveal Copper-Mediated Dioxygen Binding and Evidence for N-Terminal Deprotonation
Biochemistry ( IF 2.9 ) Pub Date : 2017-05-08 00:00:00 , DOI: 10.1021/acs.biochem.7b00019 John-Paul Bacik 1 , Sophanit Mekasha 2 , Zarah Forsberg 2 , Andrey Y. Kovalevsky 3 , Gustav Vaaje-Kolstad 2 , Vincent G. H. Eijsink 2 , Jay C. Nix 4 , Leighton Coates 3 , Matthew J. Cuneo 3 , Clifford J. Unkefer 1 , Julian C.-H. Chen 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-05-08 00:00:00 , DOI: 10.1021/acs.biochem.7b00019 John-Paul Bacik 1 , Sophanit Mekasha 2 , Zarah Forsberg 2 , Andrey Y. Kovalevsky 3 , Gustav Vaaje-Kolstad 2 , Vincent G. H. Eijsink 2 , Jay C. Nix 4 , Leighton Coates 3 , Matthew J. Cuneo 3 , Clifford J. Unkefer 1 , Julian C.-H. Chen 1
Affiliation
|
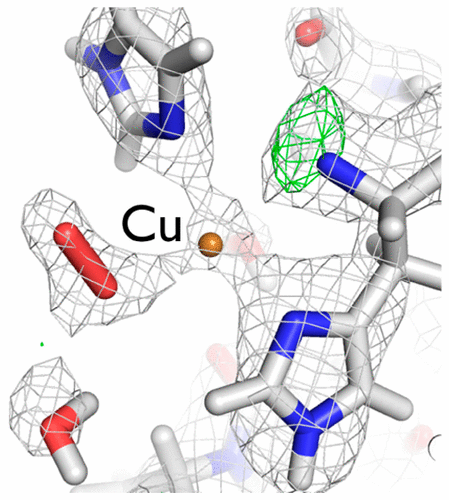
A 1.1 Å resolution, room-temperature X-ray structure and a 2.1 Å resolution neutron structure of a chitin-degrading lytic polysaccharide monooxygenase domain from the bacterium Jonesia denitrificans (JdLPMO10A) show a putative dioxygen species equatorially bound to the active site copper. Both structures show an elongated density for the dioxygen, most consistent with a Cu(II)-bound peroxide. The coordination environment is consistent with Cu(II). In the neutron and X-ray structures, difference maps reveal the N-terminal amino group, involved in copper coordination, is present as a mixed ND2 and ND–, suggesting a role for the copper ion in shifting the pKa of the amino terminus.
中文翻译:

溶解性多糖单加氧酶的中子和原子分辨率X射线结构揭示了铜介导的双氧结合和N端去质子化的证据。
细菌脱氮琼脂菌(Jd LPMO10A)的几丁质降解裂解多糖单加氧酶域的1.1分辨率,室温X射线结构和2.1分辨率中子结构显示了与活性位铜呈赤道键合的推定双氧物种。两种结构均显示出双氧的伸长密度,这与与Cu(II)结合的过氧化物最为一致。配位环境与Cu(II)一致。在中子和X射线结构中,差异图显示参与铜配位的N末端氨基以ND 2和ND –的混合形式存在,表明铜离子在改变P k a的p K a中的作用。氨基末端。
更新日期:2017-05-12
中文翻译:

溶解性多糖单加氧酶的中子和原子分辨率X射线结构揭示了铜介导的双氧结合和N端去质子化的证据。
细菌脱氮琼脂菌(Jd LPMO10A)的几丁质降解裂解多糖单加氧酶域的1.1分辨率,室温X射线结构和2.1分辨率中子结构显示了与活性位铜呈赤道键合的推定双氧物种。两种结构均显示出双氧的伸长密度,这与与Cu(II)结合的过氧化物最为一致。配位环境与Cu(II)一致。在中子和X射线结构中,差异图显示参与铜配位的N末端氨基以ND 2和ND –的混合形式存在,表明铜离子在改变P k a的p K a中的作用。氨基末端。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号