当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Staphylococcus aureus CstB Is a Novel Multidomain Persulfide Dioxygenase-Sulfurtransferase Involved in Hydrogen Sulfide Detoxification
Biochemistry ( IF 2.9 ) Pub Date : 2015-07-15 00:00:00 , DOI: 10.1021/acs.biochem.5b00584 Jiangchuan Shen , Mary E. Keithly 1 , Richard N. Armstrong 1, 2 , Khadine A. Higgins , Katherine A. Edmonds , David P. Giedroc
Biochemistry ( IF 2.9 ) Pub Date : 2015-07-15 00:00:00 , DOI: 10.1021/acs.biochem.5b00584 Jiangchuan Shen , Mary E. Keithly 1 , Richard N. Armstrong 1, 2 , Khadine A. Higgins , Katherine A. Edmonds , David P. Giedroc
Affiliation
|
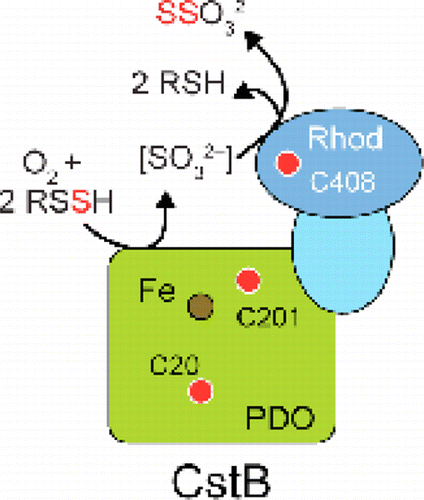
Hydrogen sulfide (H2S) is both a lethal gas and an emerging gasotransmitter in humans, suggesting that the cellular H2S level must be tightly regulated. CstB is encoded by the cst operon of the major human pathogen Staphylococcus aureus and is under the transcriptional control of the persulfide sensor CstR and H2S. Here, we show that CstB is a multifunctional Fe(II)-containing persulfide dioxygenase (PDO), analogous to the vertebrate protein ETHE1 (ethylmalonic encephalopathy protein 1). Chromosomal deletion of ethe1 is fatal in vertebrates. In the presence of molecular oxygen (O2), hETHE1 oxidizes glutathione persulfide (GSSH) to generate sulfite and reduced glutathione. In contrast, CstB oxidizes major cellular low molecular weight (LMW) persulfide substrates from S. aureus, coenzyme A persulfide (CoASSH) and bacillithiol persulfide (BSSH), directly to generate thiosulfate (TS) and reduced thiols, thereby avoiding the cellular toxicity of sulfite. Both Cys201 in the N-terminal PDO domain (CstBPDO) and Cys408 in the C-terminal rhodanese domain (CstBRhod) strongly enhance the TS generating activity of CstB. CstB also possesses persulfide transferase (PT; reverse rhodanese) activity, which generates TS when provided with LMW persulfides and sulfite, as well as conventional thiosulfate transferase (TST; rhodanese) activity; both of these activities require Cys408. CstB protects S. aureus against H2S toxicity, with the C201S and C408S cstB genes being unable to rescue a NaHS-induced ΔcstB growth phenotype. Induction of the cst operon by NaHS reveals that functional CstB impacts cellular TS concentrations. These data collectively suggest that CstB may have evolved to facilitate the clearance of LMW persulfides that occur upon elevation of the level of cellular H2S and hence may have an impact on bacterial viability under H2S misregulation, in concert with the other enzymes encoded by the cst operon.
中文翻译:

金黄色葡萄球菌CstB是涉及硫化氢解毒的新型多域过氧化物双加氧酶-硫转移酶。
硫化氢(H 2 S)在人类中既是致命气体,又是新兴的气体递质,这表明必须严格调节细胞中的H 2 S水平。CstB由主要人类病原体金黄色葡萄球菌的cst操纵子编码,并处于过硫化物传感器CstR和H 2 S的转录控制下。在这里,我们证明CstB是一种多功能的含Fe(II)的过硫化物双加氧酶(PDO)类似于脊椎动物蛋白ETHE1(乙基丙二酸脑病蛋白1)。ethe1的染色体删除在脊椎动物中是致命的。在分子氧(O 2),hETHE1会氧化谷胱甘肽过硫化物(GSSH)以生成亚硫酸盐和还原型谷胱甘肽。相比之下,CstB会氧化金黄色葡萄球菌,过氧化辅酶A(CoASSH)和过氧化硫硫醇(BSSH)的主要细胞低分子量(LMW)过硫化物底物,直接生成硫代硫酸盐(TS)和还原的硫醇,从而避免了细胞毒性。亚硫酸盐。N端PDO域(CstB PDO)中的Cys201和C端罗丹花域(CstB Rhod)中的Cys408)大大增强了CstB的TS产生活性。CstB还具有过硫转移酶(PT;反向罗丹色)活性,当与LMW过硫化物和亚硫酸盐一起提供时,会产生TS,以及常规的硫代硫酸盐转移酶(TST;罗丹色)活性;这两个活动都需要Cys408。CstB保护金黄色葡萄球菌免受H 2 S毒性,C201S和C408S cstB基因无法挽救NaHS诱导的ΔcstB生长表型。cst的归纳NaHS的operon揭示功能性CstB影响细胞TS浓度。这些数据共同表明,CstB可能已经进化为促进清除随着细胞H 2 S水平升高而发生的LMW过硫化物的清除,因此可能与其他编码的酶协同作用,对H 2 S调节异常下的细菌生存能力产生影响。由cst operon提供。
更新日期:2015-07-15
中文翻译:

金黄色葡萄球菌CstB是涉及硫化氢解毒的新型多域过氧化物双加氧酶-硫转移酶。
硫化氢(H 2 S)在人类中既是致命气体,又是新兴的气体递质,这表明必须严格调节细胞中的H 2 S水平。CstB由主要人类病原体金黄色葡萄球菌的cst操纵子编码,并处于过硫化物传感器CstR和H 2 S的转录控制下。在这里,我们证明CstB是一种多功能的含Fe(II)的过硫化物双加氧酶(PDO)类似于脊椎动物蛋白ETHE1(乙基丙二酸脑病蛋白1)。ethe1的染色体删除在脊椎动物中是致命的。在分子氧(O 2),hETHE1会氧化谷胱甘肽过硫化物(GSSH)以生成亚硫酸盐和还原型谷胱甘肽。相比之下,CstB会氧化金黄色葡萄球菌,过氧化辅酶A(CoASSH)和过氧化硫硫醇(BSSH)的主要细胞低分子量(LMW)过硫化物底物,直接生成硫代硫酸盐(TS)和还原的硫醇,从而避免了细胞毒性。亚硫酸盐。N端PDO域(CstB PDO)中的Cys201和C端罗丹花域(CstB Rhod)中的Cys408)大大增强了CstB的TS产生活性。CstB还具有过硫转移酶(PT;反向罗丹色)活性,当与LMW过硫化物和亚硫酸盐一起提供时,会产生TS,以及常规的硫代硫酸盐转移酶(TST;罗丹色)活性;这两个活动都需要Cys408。CstB保护金黄色葡萄球菌免受H 2 S毒性,C201S和C408S cstB基因无法挽救NaHS诱导的ΔcstB生长表型。cst的归纳NaHS的operon揭示功能性CstB影响细胞TS浓度。这些数据共同表明,CstB可能已经进化为促进清除随着细胞H 2 S水平升高而发生的LMW过硫化物的清除,因此可能与其他编码的酶协同作用,对H 2 S调节异常下的细菌生存能力产生影响。由cst operon提供。































 京公网安备 11010802027423号
京公网安备 11010802027423号