当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Chemical Kinetics for the Reaction of Criegee Intermediate CH2OO with HNO3 and Its Catalytic Conversion to OH and HCO
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-04-28 00:00:00 , DOI: 10.1021/acs.jpca.7b02196 P. Raghunath,Yuan-Pern Lee,M. C. Lin
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-04-28 00:00:00 , DOI: 10.1021/acs.jpca.7b02196 P. Raghunath,Yuan-Pern Lee,M. C. Lin
|
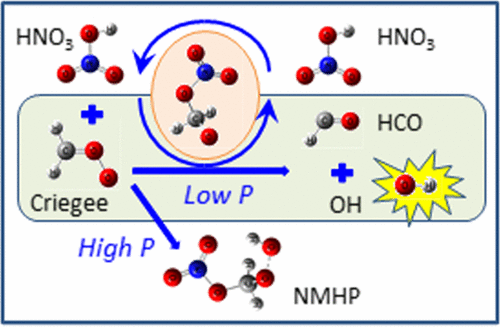
The kinetics and mechanisms for the reaction of the Criegee intermediate CH2OO with HNO3 and the unimolecular decomposition of its reaction product CH2(O)NO3 are important in atmospheric chemistry. The potential-energy profile of the reactions predicted with the CCSD(T)/aug-cc-pVTZ//B3LYP/aug-cc-pVTZ method shows that the initial association yields a prereaction complex that isomerizes by H migration to yield excited intermediate nitrooxymethyl hydroperoxide NO3CH2OOH* with internal energy ∼44 kcal mol–1. A fragmentation of this excited intermediate produces CH2(O)NO3 + OH with its transition state located 5.0 kcal mol–1 below that of the reactants. Further decomposition of CH2(O)NO3 produces HCO + HNO3, forming a catalytic cycle for destruction of CH2OO by HNO3. The rate coefficients and product-branching ratios were calculated in the temperature range 250–700 K at pressure 20–760 Torr (N2) using the variational-transition-state and Rice–Ramsperger–Kassel–Marcus (RRKM) theories. The predicted total rate coefficient for reaction CH2OO + HNO3 at 295 K, 5.1 × 10–10 cm3 molecule–1 s–1, agrees satisfactorily with the experimental value, (5.4 ± 1.0) × 10–10 cm3 molecule–1 s–1. The predicted branching ratios at 295 K are 0.21 for the formation of NO3CH2OOH and 0.79 for CH2(O)NO3 + OH at a pressure of 40 Torr (N2), and 0.79 for the formation of NO3CH2OOH and 0.21 for CH2(O)NO3 + OH at 760 Torr (N2). This new catalytic conversion of CH2OO to HCO + OH by HNO3 might have significant impact on atmospheric chemistry.
中文翻译:

Criegee中间体CH 2 OO与HNO 3的反应及其催化转化为OH和HCO的计算化学动力学
Criegee中间体CH 2 OO与HNO 3的反应动力学及其机理以及反应产物CH 2(O)NO 3的单分子分解在大气化学中很重要。用CCSD(T)/ aug-cc-pVTZ // B3LYP / aug-cc-pVTZ方法预测的反应的势能图表明,初始缔合产生预反应复合物,该复合物通过H迁移而异构化,从而生成激发的中间体硝基氧甲基内部能量约为44 kcal mol –1的氢过氧化物NO 3 CH 2 OOH * 。该被激发的中间体的碎片产生CH 2(O)NO 3+ OH的过渡态比反应物低5.0 kcal mol –1。CH 2(O)NO 3的进一步分解产生HCO + HNO 3,形成催化循环以破坏HNO 3破坏CH 2 OO 。使用变迁过渡态和莱斯-拉姆斯伯格-卡塞尔-马库斯(RRKM)理论,在250-700 K的温度范围内,压力20-760托(N 2)下计算了速率系数和产物分支比。CH 2 OO + HNO 3在295 K,5.1×10 –10 cm 3分子–1 s时的预计总速率系数–1,与实验值令人满意,(5.4±1.0)×10 –10 cm 3分子–1 s –1。在40 Torr(N 2)的压力下,对于295 K的预测支化比对于NO 3 CH 2 OOH的形成为0.21,对于CH 2(O)NO 3 + OH为0.79,对于NO 3 CH的形成为0.79。CH 2(O)NO 3 + OH在760托(N 2)下为2 OOH和0.21 。HNO 3将CH 2 OO催化转化为HCO + OH的新方法 可能会对大气化学产生重大影响。
更新日期:2017-05-11
中文翻译:

Criegee中间体CH 2 OO与HNO 3的反应及其催化转化为OH和HCO的计算化学动力学
Criegee中间体CH 2 OO与HNO 3的反应动力学及其机理以及反应产物CH 2(O)NO 3的单分子分解在大气化学中很重要。用CCSD(T)/ aug-cc-pVTZ // B3LYP / aug-cc-pVTZ方法预测的反应的势能图表明,初始缔合产生预反应复合物,该复合物通过H迁移而异构化,从而生成激发的中间体硝基氧甲基内部能量约为44 kcal mol –1的氢过氧化物NO 3 CH 2 OOH * 。该被激发的中间体的碎片产生CH 2(O)NO 3+ OH的过渡态比反应物低5.0 kcal mol –1。CH 2(O)NO 3的进一步分解产生HCO + HNO 3,形成催化循环以破坏HNO 3破坏CH 2 OO 。使用变迁过渡态和莱斯-拉姆斯伯格-卡塞尔-马库斯(RRKM)理论,在250-700 K的温度范围内,压力20-760托(N 2)下计算了速率系数和产物分支比。CH 2 OO + HNO 3在295 K,5.1×10 –10 cm 3分子–1 s时的预计总速率系数–1,与实验值令人满意,(5.4±1.0)×10 –10 cm 3分子–1 s –1。在40 Torr(N 2)的压力下,对于295 K的预测支化比对于NO 3 CH 2 OOH的形成为0.21,对于CH 2(O)NO 3 + OH为0.79,对于NO 3 CH的形成为0.79。CH 2(O)NO 3 + OH在760托(N 2)下为2 OOH和0.21 。HNO 3将CH 2 OO催化转化为HCO + OH的新方法 可能会对大气化学产生重大影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号