当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Catalytic Asymmetric Doubly Vinylogous Michael Addition of α,β‐Unsaturated γ‐Butyrolactams to Dienones
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2015-07-15 , DOI: 10.1002/anie.201504276 Xiaodong Gu , Tingting Guo , Yuanyuan Dai , Allegra Franchino , Jie Fei , Chuncheng Zou , Darren J. Dixon , Jinxing Ye
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2015-07-15 , DOI: 10.1002/anie.201504276 Xiaodong Gu , Tingting Guo , Yuanyuan Dai , Allegra Franchino , Jie Fei , Chuncheng Zou , Darren J. Dixon , Jinxing Ye
|
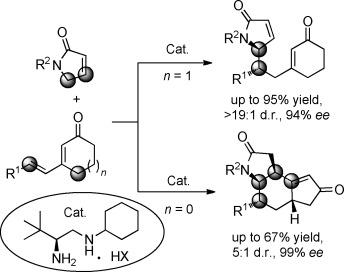
An asymmetric doubly vinylogous Michael addition (DVMA) of α,β‐unsaturated γ‐butyrolactams to sterically congested β‐substituted cyclic dienones with high site‐, diastereo‐, and enantioselectivity has been achieved. An unprecedented DVMA/vinylogous Michael addition/isomerization cascade reaction affords chiral fused tricyclic γ‐lactams with four newly formed stereocenters.
中文翻译:

α,β-不饱和γ-丁内酰胺对二烯酮的直接催化不对称双长乙烯基迈克尔加成反应
已实现了对位,非对映和对映异构体选择性高的α,β-不饱和γ-丁内酰胺向空间拥挤的β-取代的环状二烯的不对称双乙烯基迈克尔加成反应(DVMA)。前所未有的DVMA / vinyllogous Michael加成/异构化级联反应提供了具有四个新形成的立体中心的手性稠合三环γ-内酰胺。
更新日期:2015-07-15
中文翻译:

α,β-不饱和γ-丁内酰胺对二烯酮的直接催化不对称双长乙烯基迈克尔加成反应
已实现了对位,非对映和对映异构体选择性高的α,β-不饱和γ-丁内酰胺向空间拥挤的β-取代的环状二烯的不对称双乙烯基迈克尔加成反应(DVMA)。前所未有的DVMA / vinyllogous Michael加成/异构化级联反应提供了具有四个新形成的立体中心的手性稠合三环γ-内酰胺。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号