当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The total synthesis of (±)-sanggenol F
Tetrahedron ( IF 2.1 ) Pub Date : 2017-05-07 , DOI: 10.1016/j.tet.2017.05.022 Xiao Sheng , Xin-Yu Jia , Fei Tang , Yang Wang , Ai-Jun Hou
中文翻译:

(±)-桑戈尼酚F的全合成
更新日期:2017-05-07
Tetrahedron ( IF 2.1 ) Pub Date : 2017-05-07 , DOI: 10.1016/j.tet.2017.05.022 Xiao Sheng , Xin-Yu Jia , Fei Tang , Yang Wang , Ai-Jun Hou
|
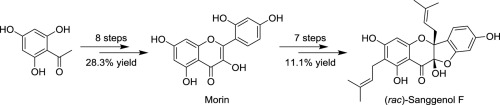
A concise and efficient total synthesis of sanggenol F (1) in racemic form has been completed via a sequence of 15 steps with an overall yield of 3.1%, starting from commercially available 2,4,6-trihydroxyacetophenone. Meanwhile, a semisynthesis of sanggenol F racemate has also been achieved in 11.1% overall yield via 7 steps with naturally-occurring morin (2) as the starting material. One step and a stepwise approach were employed to construct the two prenyl side chains at 2- and 6-positions by Claisen rearrangement reaction.
中文翻译:

(±)-桑戈尼酚F的全合成
从市售的2,4,6-三羟基苯乙酮开始,经过15个步骤的序列,已完成了外消旋形式的Sanggenol F(1)的简明有效的总合成,总收率为3.1%。同时,以天然存在的香豆素(2)为起始原料,通过7个步骤,桑格诺尔F外消旋体的半合成产率也达到了11.1%。通过克莱森重排反应,采用一步和逐步的方法在2-和6-位上构建两个异戊二烯基侧链。































 京公网安备 11010802027423号
京公网安备 11010802027423号