当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
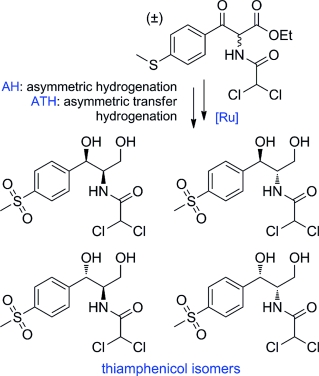
An Efficient Stereoselective Total Synthesis of All Stereoisomers of the Antibiotic Thiamphenicol through Ruthenium-Catalyzed Asymmetric Reduction by Dynamic Kinetic Resolution
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2015-07-14 , DOI: 10.1002/ejoc.201500661 Marc Perez , Pierre‐Georges Echeverria , Elsa Martinez‐Arripe , Mehdi Ez Zoubir , Ridha Touati , Zhaoguo Zhang , Jean‐Pierre Genet , Phannarath Phansavath , Tahar Ayad , Virginie Ratovelomanana‐Vidal
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2015-07-14 , DOI: 10.1002/ejoc.201500661 Marc Perez , Pierre‐Georges Echeverria , Elsa Martinez‐Arripe , Mehdi Ez Zoubir , Ridha Touati , Zhaoguo Zhang , Jean‐Pierre Genet , Phannarath Phansavath , Tahar Ayad , Virginie Ratovelomanana‐Vidal
|
Thiamphenicol is a widely used antibiotic that exhibits activity against numerous Gram-positive and Gram-negative pathogens. Here, we describe the expedient synthesis of its four stereoisomers through a dynamic kinetic resolution that follows a ruthenium-catalyzed asymmetric hydrogenation or a hydrogen transfer reaction as the key step.
中文翻译:

通过动态动力学拆分通过钌催化的不对称还原有效立体选择性地全合成抗生素噻吩菌素的所有立体异构体
硫霉素是一种广泛使用的抗生素,对多种革兰氏阳性和革兰氏阴性病原体具有活性。在这里,我们通过动态动力学拆分描述了其四种立体异构体的便利合成,该拆分遵循钌催化的不对称氢化或氢转移反应作为关键步骤。
更新日期:2015-07-14
中文翻译:

通过动态动力学拆分通过钌催化的不对称还原有效立体选择性地全合成抗生素噻吩菌素的所有立体异构体
硫霉素是一种广泛使用的抗生素,对多种革兰氏阳性和革兰氏阴性病原体具有活性。在这里,我们通过动态动力学拆分描述了其四种立体异构体的便利合成,该拆分遵循钌催化的不对称氢化或氢转移反应作为关键步骤。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号