当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Li+ Defects in a Solid-State Li Ion Battery: Theoretical Insights with a Li3OCl Electrolyte
Chemistry of Materials ( IF 7.2 ) Pub Date : 2017-04-26 00:00:00 , DOI: 10.1021/acs.chemmater.7b00659 Saskia Stegmaier 1 , Johannes Voss 2 , Karsten Reuter 3 , Alan C. Luntz 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2017-04-26 00:00:00 , DOI: 10.1021/acs.chemmater.7b00659 Saskia Stegmaier 1 , Johannes Voss 2 , Karsten Reuter 3 , Alan C. Luntz 2
Affiliation
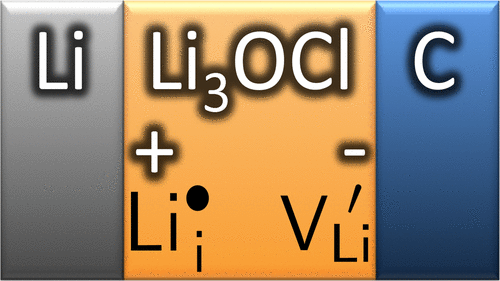
|
In a solid-state Li ion battery, the solid-state electrolyte exists principally in regions of high externally applied potentials, and this varies rapidly at the interfaces with electrodes because of the formation of electrochemical double layers. We investigate the implications of these for a model solid-state Li ion Li|Li3OCl|C battery, where C is simply a metallic intercalation cathode. We use density functional theory to calculate the potential dependence of the formation energies of the Li+ charge carriers in superionic Li3OCl. We find that Li+ vacancies are the dominant species at the cathode while Li+ interstitials dominate at the anode. With typical Mg aliovalent doping of Li3OCl, Li+ vacancies dominate the bulk of the electrolyte, as well, with freely mobile vacancies that are only ∼10–4 of the Mg doping density at room temperature. We study the repulsive interaction between Li+ vacancies and find that this is extremely short-range, typically only one lattice constant because of local structural relaxation around the vacancy, and this is significantly shorter than pure electrostatic screening. We model a Li3OCl–cathode interface by treating the cathode as a nearly ideal metal using a polarizable continuum model with an εr of 1000. There is a large interface segregation free energy of approximately −1 eV per Li+ vacancy. Combined with the short range for repulsive interactions of the vacancies, this means that very large vacancy concentrations will build up in a single layer of Li3OCl at the cathode interface to form a compact double layer. The calculated potential drop across the interface is ∼3 V for a nearly full concentration of vacancies at the surface. This suggests that nearly all the cathode potential drop in Li3OCl occurs at the Helmholtz plane rather than in a diffuse space–charge region. We suggest that our conclusions can be generalized to other superionic conductors, as well.
中文翻译:

固态锂离子电池中的Li +缺陷:Li 3 OCl电解质的理论见解
在固态锂离子电池中,固态电解质主要存在于高外部施加电势的区域中,并且由于形成电化学双层而在与电极的界面处迅速变化。我们研究了这些对于模型固态Li离子Li | Li 3 OCl | C电池的影响,其中C只是金属插入阴极。我们使用密度泛函理论来计算超离子Li 3 OCl中Li +电荷载流子形成能的电势依赖性。我们发现,锂+空缺为优势种在阴极而李+插页式独领风骚的阳极。具有典型的Li 3镁异价掺杂OCl,Li +空位也占主导地位,在室温下,自由移动空位仅为Mg掺杂密度的约10 –4。我们研究了Li +空位之间的排斥相互作用,发现这是极短程的,由于空位周围的局部结构松弛,通常只有一个晶格常数,这比纯静电筛选要短得多。我们使用εr为1000的可极化连续体模型,通过将阴极视为接近理想的金属来对Li 3 OCl-阴极界面进行建模。每个Li +都有大约-1 eV的大界面离析自由能空缺。结合空位的排斥相互作用的短距离,这意味着非常大的空位浓度将在阴极界面的单层Li 3 OCl中积聚,从而形成紧凑的双层。对于表面上几乎完全的空位浓度,通过界面计算得出的电位降约为3V。这表明Li 3 OCl中几乎所有的阴极电位下降都发生在亥姆霍兹平面上,而不是在扩散的空间电荷区域内。我们建议我们的结论也可以推广到其他超离子导体。
更新日期:2017-05-05
中文翻译:

固态锂离子电池中的Li +缺陷:Li 3 OCl电解质的理论见解
在固态锂离子电池中,固态电解质主要存在于高外部施加电势的区域中,并且由于形成电化学双层而在与电极的界面处迅速变化。我们研究了这些对于模型固态Li离子Li | Li 3 OCl | C电池的影响,其中C只是金属插入阴极。我们使用密度泛函理论来计算超离子Li 3 OCl中Li +电荷载流子形成能的电势依赖性。我们发现,锂+空缺为优势种在阴极而李+插页式独领风骚的阳极。具有典型的Li 3镁异价掺杂OCl,Li +空位也占主导地位,在室温下,自由移动空位仅为Mg掺杂密度的约10 –4。我们研究了Li +空位之间的排斥相互作用,发现这是极短程的,由于空位周围的局部结构松弛,通常只有一个晶格常数,这比纯静电筛选要短得多。我们使用εr为1000的可极化连续体模型,通过将阴极视为接近理想的金属来对Li 3 OCl-阴极界面进行建模。每个Li +都有大约-1 eV的大界面离析自由能空缺。结合空位的排斥相互作用的短距离,这意味着非常大的空位浓度将在阴极界面的单层Li 3 OCl中积聚,从而形成紧凑的双层。对于表面上几乎完全的空位浓度,通过界面计算得出的电位降约为3V。这表明Li 3 OCl中几乎所有的阴极电位下降都发生在亥姆霍兹平面上,而不是在扩散的空间电荷区域内。我们建议我们的结论也可以推广到其他超离子导体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号