当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triggering Redox Activity in a Thiophene Compound: Radical Stabilization and Coordination Chemistry
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-06-01 , DOI: 10.1002/anie.201703576 Massimiliano Curcio 1 , James R. Pankhurst 1 , Stephen Sproules 2 , Dimitri Mignard 1 , Jason B. Love 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-06-01 , DOI: 10.1002/anie.201703576 Massimiliano Curcio 1 , James R. Pankhurst 1 , Stephen Sproules 2 , Dimitri Mignard 1 , Jason B. Love 1
Affiliation
|
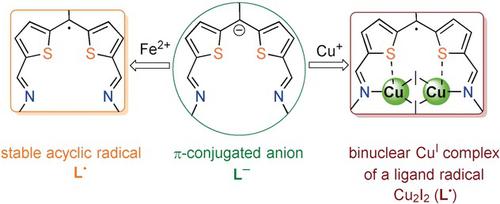
The synthesis, metalation, and redox properties of an acyclic bis(iminothienyl)methene L− are presented. This π‐conjugated anion displayed pronounced redox activity, undergoing facile one‐electron oxidation to the acyclic, metal‐free, neutral radical L. on reaction with FeBr2. In contrast, the reaction of L− with CuI formed the unique, neutral Cu2I2(L.) complex of a ligand‐centered radical, whereas reaction with the stronger oxidant AgBF4 formed the metal‐free radical dication L.2+.
中文翻译:

触发噻吩化合物中的氧化还原活性:自由基稳定和配位化学。
合成,无环双(iminothienyl)亚甲基的金属化和氧化还原性质大号-被呈现。这种π共轭阴离子显示出明显的氧化还原活性,对无环,无金属的中性基团L进行了容易的单电子氧化。与FeBr 2反应。相比之下,反应大号-用的CuI形成了独特的,中性的Cu 2我2(大号。)络合物的配体为中心的基团,而具有更强的氧化剂的AgBF反应4所形成的不含金属的自由基二价阳离子大号.2+。
更新日期:2017-06-01
中文翻译:

触发噻吩化合物中的氧化还原活性:自由基稳定和配位化学。
合成,无环双(iminothienyl)亚甲基的金属化和氧化还原性质大号-被呈现。这种π共轭阴离子显示出明显的氧化还原活性,对无环,无金属的中性基团L进行了容易的单电子氧化。与FeBr 2反应。相比之下,反应大号-用的CuI形成了独特的,中性的Cu 2我2(大号。)络合物的配体为中心的基团,而具有更强的氧化剂的AgBF反应4所形成的不含金属的自由基二价阳离子大号.2+。































 京公网安备 11010802027423号
京公网安备 11010802027423号