当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
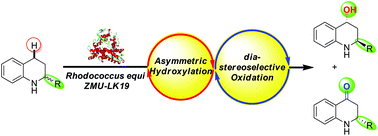
Enantioselective synthesis of 1,2,3,4-tetrahydroquinoline-4-ols and 2,3-dihydroquinolin-4(1H)-ones via a sequential asymmetric hydroxylation/diastereoselective oxidation process using Rhodococcus equi ZMU-LK19
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-01-30 00:00:00 , DOI: 10.1039/c7ob00151g Ke Li 1, 2, 3, 4, 5 , Juxiang Wang 1, 2, 3, 4, 5 , Kailin Wu 1, 2, 3, 4, 5 , Daijun Zheng 1, 2, 3, 4, 5 , Xiaojian Zhou 1, 2, 3, 4, 5 , Wenyong Han 1, 2, 3, 4, 5 , Nanwei Wan 1, 2, 3, 4, 5 , Baodong Cui 1, 2, 3, 4, 5 , Yongzheng Chen 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-01-30 00:00:00 , DOI: 10.1039/c7ob00151g Ke Li 1, 2, 3, 4, 5 , Juxiang Wang 1, 2, 3, 4, 5 , Kailin Wu 1, 2, 3, 4, 5 , Daijun Zheng 1, 2, 3, 4, 5 , Xiaojian Zhou 1, 2, 3, 4, 5 , Wenyong Han 1, 2, 3, 4, 5 , Nanwei Wan 1, 2, 3, 4, 5 , Baodong Cui 1, 2, 3, 4, 5 , Yongzheng Chen 1, 2, 3, 4, 5
Affiliation
|
A cascade biocatalysis system involving asymmetric hydroxylation and diastereoselective oxidation was developed using Rhodococcus equi ZMU-LK19, which gave chiral 2-substituted-1,2,3,4-tetrahydroquinoline-4-ols (2) (up to 57% isolated yield, 99 :
: 1 dr, and >99% ee) and chiral 2-substituted-2,3-dihydroquinolin-4(1H)-ones (3) (up to 25% isolated yield, and >99% ee) from (±)-2-substituted-tetrahydroquinolines (1). In addition, a possible mechanism for this cascade biocatalysis was tentatively proposed.
1 dr, and >99% ee) and chiral 2-substituted-2,3-dihydroquinolin-4(1H)-ones (3) (up to 25% isolated yield, and >99% ee) from (±)-2-substituted-tetrahydroquinolines (1). In addition, a possible mechanism for this cascade biocatalysis was tentatively proposed.
中文翻译:

对映选择性合成1,2,3,4-四氢喹啉-4-醇和2,3-二氢喹啉-4(1 H)-酮,通过连续不对称羟基化/非对映选择性氧化工艺使用马氏红球菌ZMU-LK19
使用马生红球菌ZMU-LK19开发了一种涉及不对称羟基化和非对映选择性氧化的级联生物催化系统,该系统可提供手性2-取代-1,2,3,4-四氢喹啉-4-醇(2)(分离产率高达57%, 99 :
: 1 dr,> 99%ee)和(± )的手性2-取代-2,3-二氢喹啉-4(1 H)-ones(3)(分离产率最高25%,ee> 99%) )-2-取代的四氢喹啉(1)。另外,初步提出了这种级联生物催化的可能机制。
1 dr,> 99%ee)和(± )的手性2-取代-2,3-二氢喹啉-4(1 H)-ones(3)(分离产率最高25%,ee> 99%) )-2-取代的四氢喹啉(1)。另外,初步提出了这种级联生物催化的可能机制。
更新日期:2017-05-03
 :
: 1 dr, and >99% ee) and chiral 2-substituted-2,3-dihydroquinolin-4(1H)-ones (3) (up to 25% isolated yield, and >99% ee) from (±)-2-substituted-tetrahydroquinolines (1). In addition, a possible mechanism for this cascade biocatalysis was tentatively proposed.
1 dr, and >99% ee) and chiral 2-substituted-2,3-dihydroquinolin-4(1H)-ones (3) (up to 25% isolated yield, and >99% ee) from (±)-2-substituted-tetrahydroquinolines (1). In addition, a possible mechanism for this cascade biocatalysis was tentatively proposed.
中文翻译:

对映选择性合成1,2,3,4-四氢喹啉-4-醇和2,3-二氢喹啉-4(1 H)-酮,通过连续不对称羟基化/非对映选择性氧化工艺使用马氏红球菌ZMU-LK19
使用马生红球菌ZMU-LK19开发了一种涉及不对称羟基化和非对映选择性氧化的级联生物催化系统,该系统可提供手性2-取代-1,2,3,4-四氢喹啉-4-醇(2)(分离产率高达57%, 99
 :
: 1 dr,> 99%ee)和(± )的手性2-取代-2,3-二氢喹啉-4(1 H)-ones(3)(分离产率最高25%,ee> 99%) )-2-取代的四氢喹啉(1)。另外,初步提出了这种级联生物催化的可能机制。
1 dr,> 99%ee)和(± )的手性2-取代-2,3-二氢喹啉-4(1 H)-ones(3)(分离产率最高25%,ee> 99%) )-2-取代的四氢喹啉(1)。另外,初步提出了这种级联生物催化的可能机制。















































 京公网安备 11010802027423号
京公网安备 11010802027423号