当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Discovery of 4-(6-Methoxy-2-methyl-4-(quinolin-4-yl)-9H-pyrimido[4,5-b]indol-7-yl)-3,5-dimethylisoxazole (CD161) as a Potent and Orally Bioavailable BET Bromodomain Inhibitor.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-02 , DOI: 10.1021/acs.jmedchem.7b00193 Yujun Zhao 1 , Longchuan Bai 1 , Liu Liu 1 , Donna McEachern 1 , Jeanne A Stuckey 2 , Jennifer L Meagher 2 , Chao-Yie Yang 1 , Xu Ran 1 , Bing Zhou 1 , Yang Hu 1 , Xiaoqin Li 3 , Bo Wen 3 , Ting Zhao 3 , Siwei Li 3 , Duxin Sun 3 , Shaomeng Wang 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-02 , DOI: 10.1021/acs.jmedchem.7b00193 Yujun Zhao 1 , Longchuan Bai 1 , Liu Liu 1 , Donna McEachern 1 , Jeanne A Stuckey 2 , Jennifer L Meagher 2 , Chao-Yie Yang 1 , Xu Ran 1 , Bing Zhou 1 , Yang Hu 1 , Xiaoqin Li 3 , Bo Wen 3 , Ting Zhao 3 , Siwei Li 3 , Duxin Sun 3 , Shaomeng Wang 1
Affiliation
|
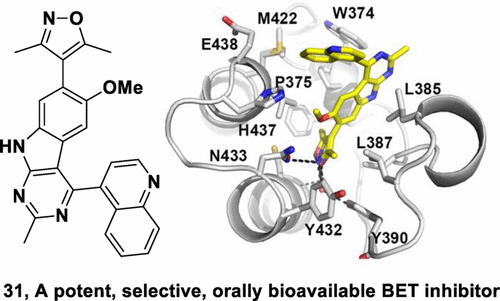
We have designed and synthesized 9H-pyrimido[4,5-b]indole-containing compounds to obtain potent and orally bioavailable BET inhibitors. By incorporation of an indole or a quinoline moiety to the 9H-pyrimido[4,5-b]indole core, we identified a series of small molecules showing high binding affinities to BET proteins and low nanomolar potencies in inhibition of cell growth in acute leukemia cell lines. One such compound, 4-(6-methoxy-2-methyl-4-(quinolin-4-yl)-9H-pyrimido[4,5-b]indol-7-yl)-3,5-dimethylisoxazole (31) has excellent microsomal stability and good oral pharmacokinetics in rats and mice. Orally administered, 31 achieves significant antitumor activity in the MV4;11 leukemia and MDA-MB-231 triple-negative breast cancer xenograft models in mice. Determination of the cocrystal structure of 31 with BRD4 BD2 provides a structural basis for its high binding affinity to BET proteins. Testing its binding affinities against other bromodomain-containing proteins shows that 31 is a highly selective inhibitor of BET proteins. Our data show that 31 is a potent, selective, and orally active BET inhibitor.
中文翻译:

基于结构的4-(6-甲氧基-2-甲基-4-(喹啉-4-基)-9H-嘧啶[4,5-b]吲哚-7-基)-3,5-二甲基异恶唑(CD161 )作为有效的和口服生物可利用的BET溴结构域抑制剂。
我们设计并合成了含9H-嘧啶[4,5-b]吲哚的化合物,以获得有效的和口服可生物利用的BET抑制剂。通过将吲哚或喹啉部分掺入9H-嘧啶[4,5-b]吲哚核中,我们鉴定了一系列小分子,它们对BET蛋白显示出高结合亲和力,并且在抑制急性白血病细胞生长方面具有低纳摩尔浓度。细胞系。一种这样的化合物4-(6-甲氧基-2-甲基-4-(喹啉-4-基)-9H-嘧啶[4,5-b]吲哚-7-基)-3,5-二甲基异恶唑(31)在大鼠和小鼠中具有出色的微粒体稳定性和良好的口服药代动力学。口服31在小鼠的MV4; 11白血病和MDA-MB-231三阴性乳腺癌异种移植模型中实现了显着的抗肿瘤活性。用BRD4 BD2鉴定31的共晶体结构为其与BET蛋白的高结合亲和力提供了结构基础。测试其与其他含溴结构域蛋白的结合亲和力表明31是BET蛋白的高度选择性抑制剂。我们的数据表明31是一种有效的,选择性的和口服活性的BET抑制剂。
更新日期:2017-05-02
中文翻译:

基于结构的4-(6-甲氧基-2-甲基-4-(喹啉-4-基)-9H-嘧啶[4,5-b]吲哚-7-基)-3,5-二甲基异恶唑(CD161 )作为有效的和口服生物可利用的BET溴结构域抑制剂。
我们设计并合成了含9H-嘧啶[4,5-b]吲哚的化合物,以获得有效的和口服可生物利用的BET抑制剂。通过将吲哚或喹啉部分掺入9H-嘧啶[4,5-b]吲哚核中,我们鉴定了一系列小分子,它们对BET蛋白显示出高结合亲和力,并且在抑制急性白血病细胞生长方面具有低纳摩尔浓度。细胞系。一种这样的化合物4-(6-甲氧基-2-甲基-4-(喹啉-4-基)-9H-嘧啶[4,5-b]吲哚-7-基)-3,5-二甲基异恶唑(31)在大鼠和小鼠中具有出色的微粒体稳定性和良好的口服药代动力学。口服31在小鼠的MV4; 11白血病和MDA-MB-231三阴性乳腺癌异种移植模型中实现了显着的抗肿瘤活性。用BRD4 BD2鉴定31的共晶体结构为其与BET蛋白的高结合亲和力提供了结构基础。测试其与其他含溴结构域蛋白的结合亲和力表明31是BET蛋白的高度选择性抑制剂。我们的数据表明31是一种有效的,选择性的和口服活性的BET抑制剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号