当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tertiary Mg/MgCl2/AlCl3 Inorganic Mg2+ Electrolytes with Unprecedented Electrochemical Performance for Reversible Mg Deposition
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-04-24 00:00:00 , DOI: 10.1021/acsenergylett.7b00269
Jian Luo 1 , Shuijian He 1 , T. Leo Liu 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-04-24 00:00:00 , DOI: 10.1021/acsenergylett.7b00269
Jian Luo 1 , Shuijian He 1 , T. Leo Liu 1
Affiliation
|
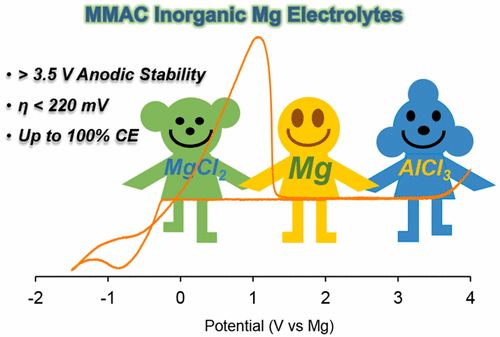
Because of the high reactivity and sensitivity of Mg2+ electrolytes in organic solvents, developing facile methods of preparing high-performance Mg2+ electrolytes is still challenging and thus impedes the development of Mg batteries. In this study, a convenient method involving tertiary reactants, Mg powder, MgCl2, and AlCl3, was reported to prepare all-inorganic Mg2+ electrolytes (termed MMAC electrolytes) in ethereal solvents. These MMAC electrolytes exhibited unprecedented performance for reversible Mg deposition, Coulombic efficiencies at 90–100%, overpotential of 125–215 mV, and anodic stability up to 3.5–3.8 V (vs Mg). A comprehensive fundamental study of the MMAC electrolytes showed that the electron transfer and mass transport kinetics during Mg deposition and stripping were affected by solvent, working electrode, and the composition of the electrolytes. In brief, these tertiary MMAC electrolytes represent the most facile and reliable inorganic Mg electrolytes known to date.
中文翻译:

具有前所未有的电化学性能的Mg / MgCl 2 / AlCl 3无机Mg 2+电解质可逆沉积Mg
由于Mg 2+电解质在有机溶剂中的高反应活性和敏感性,开发制备高性能Mg 2+电解质的简便方法仍然具有挑战性,因此阻碍了Mg电池的发展。在这项研究中,据报道,一种方便的方法涉及叔反应物,Mg粉末,MgCl 2和AlCl 3,可在醚溶剂中制备全无机Mg 2+电解质(称为MMAC电解质)。这些MMAC电解质在可逆的Mg沉积方面表现出空前的性能,库仑效率在90-100%,过电势在125-215 mV,阳极稳定性高达3.5-3.8 V(vs Mg)。对MMAC电解质的全面基础研究表明,在Mg沉积和汽提过程中,电子转移和质量传输动力学受溶剂,工作电极和电解质组成的影响。简而言之,这些叔MMAC电解质代表了迄今为止已知的最便捷,最可靠的无机Mg电解质。
更新日期:2017-05-03
中文翻译:

具有前所未有的电化学性能的Mg / MgCl 2 / AlCl 3无机Mg 2+电解质可逆沉积Mg
由于Mg 2+电解质在有机溶剂中的高反应活性和敏感性,开发制备高性能Mg 2+电解质的简便方法仍然具有挑战性,因此阻碍了Mg电池的发展。在这项研究中,据报道,一种方便的方法涉及叔反应物,Mg粉末,MgCl 2和AlCl 3,可在醚溶剂中制备全无机Mg 2+电解质(称为MMAC电解质)。这些MMAC电解质在可逆的Mg沉积方面表现出空前的性能,库仑效率在90-100%,过电势在125-215 mV,阳极稳定性高达3.5-3.8 V(vs Mg)。对MMAC电解质的全面基础研究表明,在Mg沉积和汽提过程中,电子转移和质量传输动力学受溶剂,工作电极和电解质组成的影响。简而言之,这些叔MMAC电解质代表了迄今为止已知的最便捷,最可靠的无机Mg电解质。

































 京公网安备 11010802027423号
京公网安备 11010802027423号