当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hierarchical NiCo2S4@NiFe LDH Heterostructures Supported on Nickel Foam for Enhanced Overall-Water-Splitting Activity
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-04-28 00:00:00 , DOI: 10.1021/acsami.7b00019 Jia Liu 1 , Jinsong Wang 1 , Bao Zhang 1 , Yunjun Ruan 1 , Lin Lv 1 , Xiao Ji 1 , Kui Xu 1 , Ling Miao 1 , Jianjun Jiang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-04-28 00:00:00 , DOI: 10.1021/acsami.7b00019 Jia Liu 1 , Jinsong Wang 1 , Bao Zhang 1 , Yunjun Ruan 1 , Lin Lv 1 , Xiao Ji 1 , Kui Xu 1 , Ling Miao 1 , Jianjun Jiang 1
Affiliation
|
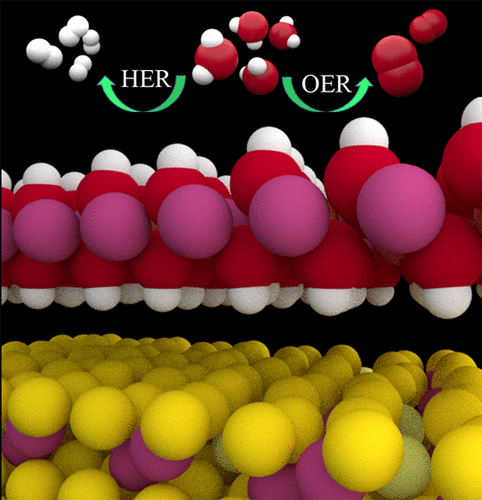
Low-cost and highly efficient bifunctional electrocatalysts for the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) are intensively investigated for overall water splitting. Herein, we combined experimental research with first-principles calculations based on density functional theory (DFT) to engineer the NiCo2S4@NiFe LDH heterostructure interface for enhancing overall water-splitting activity. The DFT calculations exhibit strong interaction and charge transfer between NiCo2S4 and NiFe LDH, which change the interfacial electronic structure and surface reactivity. The calculated chemisorption free energy of hydroxide (ΔEOH) is reduced from 1.56 eV for pure NiFe LDH to 1.03 eV for the heterostructures, indicating a dramatic improvement in OER performance, while the chemisorption free energy of hydrogen (ΔEH) maintains almost invariable. By the use of the facile hydrothermal method, NiCo2S4 nanotubes, NiFe LDH nanosheets, and NiCo2S4@NiFe LDH heterostructures are prepared on nickel foam, of which the corresponding experimental OER overpotentials are 306, 260, and 201 mV at 60 mA cm–2, respectively. These results are good agreement with the theoretical predictions. Meanwhile, the HER performance has little improvement, with an overpotential of about 200 mV at 10 mA cm–2. Due to the dramatic improvement in OER performance, there was an enhancement in the overall water-splitting activity of the NiCo2S4@NiFe LDH heterostructures, with a low voltage of 1.6 V.
中文翻译:

NiCo 2 S 4 @NiFe LDH分层结构支撑在镍泡沫上以增强总水分解活性
深入研究了用于氢气析出反应(HER)和氧气析出反应(OER)的低成本,高效双功能电催化剂,以进行整体的水分解。在这里,我们将实验研究与基于密度泛函理论(DFT)的第一性原理计算相结合,以设计NiCo 2 S 4 @NiFe LDH异质结构界面,以增强整体的水分解活性。DFT计算显示出NiCo 2 S 4和NiFe LDH之间的强相互作用和电荷转移,这改变了界面电子结构和表面反应性。计算的氢氧化物化学吸附自由能(ΔE OH)从纯NiFe LDH的1.56 eV降低到异质结构的1.03 eV,这表明OER性能有了显着提高,而氢的化学吸附自由能(ΔE H)几乎保持不变。通过使用方便的水热法,在泡沫镍上制备了NiCo 2 S 4纳米管,NiFe LDH纳米片和NiCo 2 S 4 @NiFe LDH异质结构,其中相应的实验OER超电势为306、260和201 mV。分别为60 mA cm –2。这些结果与理论预测吻合良好。同时,HER性能几乎没有改善,在10 mA cm –2时的过电势约为200 mV。由于OER性能的显着改善,在低电压1.6 V的情况下,NiCo 2 S 4 @NiFe LDH异质结构的总水分解活性得到了增强。
更新日期:2017-04-28
中文翻译:

NiCo 2 S 4 @NiFe LDH分层结构支撑在镍泡沫上以增强总水分解活性
深入研究了用于氢气析出反应(HER)和氧气析出反应(OER)的低成本,高效双功能电催化剂,以进行整体的水分解。在这里,我们将实验研究与基于密度泛函理论(DFT)的第一性原理计算相结合,以设计NiCo 2 S 4 @NiFe LDH异质结构界面,以增强整体的水分解活性。DFT计算显示出NiCo 2 S 4和NiFe LDH之间的强相互作用和电荷转移,这改变了界面电子结构和表面反应性。计算的氢氧化物化学吸附自由能(ΔE OH)从纯NiFe LDH的1.56 eV降低到异质结构的1.03 eV,这表明OER性能有了显着提高,而氢的化学吸附自由能(ΔE H)几乎保持不变。通过使用方便的水热法,在泡沫镍上制备了NiCo 2 S 4纳米管,NiFe LDH纳米片和NiCo 2 S 4 @NiFe LDH异质结构,其中相应的实验OER超电势为306、260和201 mV。分别为60 mA cm –2。这些结果与理论预测吻合良好。同时,HER性能几乎没有改善,在10 mA cm –2时的过电势约为200 mV。由于OER性能的显着改善,在低电压1.6 V的情况下,NiCo 2 S 4 @NiFe LDH异质结构的总水分解活性得到了增强。

































 京公网安备 11010802027423号
京公网安备 11010802027423号