当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity Comparison of Primary Aromatic Amines and Thiols in E–H Insertion Reactions with Diazoacetates Catalyzed by Iridium(III) Tetratolylporphyrin
Organometallics ( IF 2.5 ) Pub Date : 2017-04-28 00:00:00 , DOI: 10.1021/acs.organomet.7b00185 Bernie J. Anding 1 , Taiwo O. Dairo 1 , L. Keith Woo 1
Organometallics ( IF 2.5 ) Pub Date : 2017-04-28 00:00:00 , DOI: 10.1021/acs.organomet.7b00185 Bernie J. Anding 1 , Taiwo O. Dairo 1 , L. Keith Woo 1
Affiliation
|
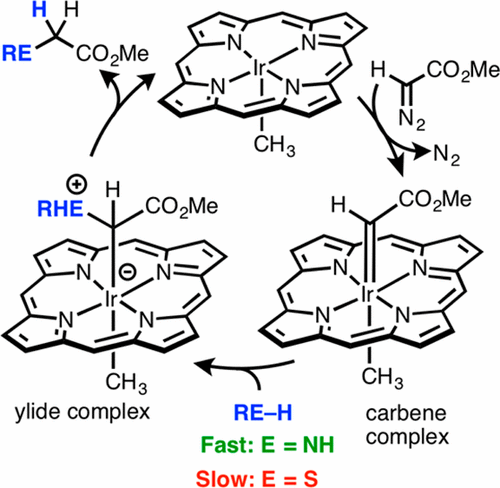
A detailed kinetic study is described for the insertion of carbenes from methyl diazoacetate into the N–H bond of aniline, using Ir(TTP)CH3 (TTP = tetratolylporphyrinato) as a catalyst. Aniline strongly coordinates to the Ir center with a binding constant of K = (2.5 ± 0.5) × 104 at 296 K, forming an inactive hexacoordinate complex, (aniline)Ir(TTP)CH3. The rate of N–H insertion is first order in both diazo ester and catalyst. When the true amount of active, five-coordinate Ir(TTP)CH3 is taken into account, the insertion rate is found to be independent of the aniline concentration. This indicates that the rate-limiting step in the catalytic cycle occurs prior to the nucleophilic attack of aniline on an Ir carbene complex to generate a coordinated ylide. Thus, with N–H insertion catalyzed by Ir(TTP)CH3, aniline is both a strong ligand and a potent nucleophile. This is in contrast to the analogous catalytic insertion of carbenes into S–H bonds. p-Toluenethiol is a more weakly binding ligand toward Ir(TTP)CH3 (K = 680 ± 20 at 296 K). Moreover, the rate of S–H insertion is first order in diazo reagent, catalyst, and thiol concentrations. In this case, the slow step is nucleophilic attack of the thiol on the Ir carbene complex to form a coordinated sulfonium ylide intermediate. In comparison to aniline, p-toluenethiol is a weaker ligand and a poorer nucleophile. The consequence of these differences is that the rate of aniline attack on the carbene intermediate is much faster than the rate of formation of the intermediate carbene complex; whereas the rate of nucleophilic addition of the thiol is slower that the rate of carbene complex formation.
中文翻译:

四甲苯基卟啉铱(III)催化重氮乙酸酯在E–H插入反应中伯芳香胺和硫醇的反应性比较
使用Ir(TTP)CH 3(TTP = tetratolylporphyrinato)作为催化剂,详细描述了将重氮乙酸甲酯中的卡宾插入苯胺的N–H键的动力学研究。苯胺在296 K处的结合常数为K =(2.5±0.5)×10 4,从而强烈地与Ir中心配位,形成无活性的六坐标络合物(苯胺)Ir(TTP)CH 3。在重氮酯和催化剂中,NH的插入速率均为一阶。当有效量为五坐标的Ir(TTP)CH 3时如果考虑到苯甲酰胺的浓度,则发现插入速率与苯胺浓度无关。这表明催化循环中的限速步骤发生在苯胺对Ir卡宾络合物的亲核攻击以生成配位的叶立德之前。因此,通过Ir(TTP)CH 3催化的N–H插入,苯胺既是强配体又是强亲核试剂。与之相反的是,羰基碳氢化合物类似地催化插入到S–H键中。对甲苯硫醇是对Ir(TTP)CH 3(K=在296 K时为680±20)。此外,在重氮试剂,催化剂和硫醇浓度中,S–H的插入速率是一阶的。在这种情况下,缓慢的步骤是硫醇对Ir卡宾络合物的亲核攻击,形成配位的sulf叶立德中间体。与苯胺相比,对甲苯硫醇是较弱的配体和较差的亲核试剂。这些差异的结果是苯胺对卡宾中间体的攻击速率比中间体卡宾络合物的生成速率要快得多。巯基的亲核加成速率比卡宾配合物形成速率慢。
更新日期:2017-04-29
中文翻译:

四甲苯基卟啉铱(III)催化重氮乙酸酯在E–H插入反应中伯芳香胺和硫醇的反应性比较
使用Ir(TTP)CH 3(TTP = tetratolylporphyrinato)作为催化剂,详细描述了将重氮乙酸甲酯中的卡宾插入苯胺的N–H键的动力学研究。苯胺在296 K处的结合常数为K =(2.5±0.5)×10 4,从而强烈地与Ir中心配位,形成无活性的六坐标络合物(苯胺)Ir(TTP)CH 3。在重氮酯和催化剂中,NH的插入速率均为一阶。当有效量为五坐标的Ir(TTP)CH 3时如果考虑到苯甲酰胺的浓度,则发现插入速率与苯胺浓度无关。这表明催化循环中的限速步骤发生在苯胺对Ir卡宾络合物的亲核攻击以生成配位的叶立德之前。因此,通过Ir(TTP)CH 3催化的N–H插入,苯胺既是强配体又是强亲核试剂。与之相反的是,羰基碳氢化合物类似地催化插入到S–H键中。对甲苯硫醇是对Ir(TTP)CH 3(K=在296 K时为680±20)。此外,在重氮试剂,催化剂和硫醇浓度中,S–H的插入速率是一阶的。在这种情况下,缓慢的步骤是硫醇对Ir卡宾络合物的亲核攻击,形成配位的sulf叶立德中间体。与苯胺相比,对甲苯硫醇是较弱的配体和较差的亲核试剂。这些差异的结果是苯胺对卡宾中间体的攻击速率比中间体卡宾络合物的生成速率要快得多。巯基的亲核加成速率比卡宾配合物形成速率慢。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号