当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Need for an Alternative to Radicals as the Cause of Fragmentation of a Thiamin-Derived Breslow Intermediate.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-May-22 , DOI: 10.1002/anie.201702240 Michael Bielecki 1 , Ronald Kluger 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-May-22 , DOI: 10.1002/anie.201702240 Michael Bielecki 1 , Ronald Kluger 1
Affiliation
|
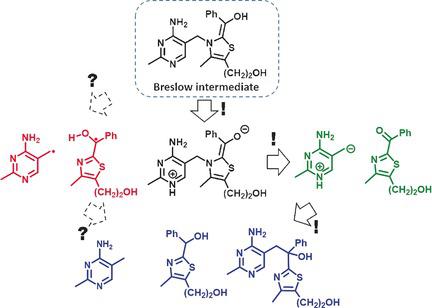
Mandelylthiamin (1) is a conjugate of benzoylformate and thiamin that loses CO2 to form the classic Breslow intermediate (2), whose expected fate is formation of the thiamin conjugate of benzaldehyde (3). Surprisingly, it was observed that 2 decomposes to 4 and 5 and rearranges to 6 in competition with the expected protonation to give 3. Recent reports propose that the alternatives to protonation arise from homolysis followed by radical-centered processes. It is now found, instead, that the spectroscopic observations cited in support of the proposed radical pathways are likely to be the result of other events. An alternative explanation is that ionization of the enolic hydroxy group of 2 and resultant electronic reorganization leads to C-C bond cleavage and non-radical intermediates that readily form 4, 5, and 6.
中文翻译:

需要替代自由基作为硫胺衍生的布雷斯洛中间体断裂的原因。
曼荼罗硫胺素(1)是苯甲酰甲酸酯和硫胺素的共轭物,会损失CO 2形成经典的Breslow中间体(2),其预期结果是形成苯甲醛的硫胺素共轭物(3)。出乎意料的是,观察到2与预期的质子化竞争而分解为4和5,并重排为6,从而得到3。最近的报告提出质子化的替代方法来自均质化,然后是自由基为中心的过程。现在发现,为支持所提出的自由基途径而引用的光谱观察结果很可能是其他事件的结果。另一种解释是2的烯醇羟基的电离和产生的电子重组会导致CC键断裂和易于形成4、5和6的非自由基中间体。
更新日期:2017-04-29
中文翻译:

需要替代自由基作为硫胺衍生的布雷斯洛中间体断裂的原因。
曼荼罗硫胺素(1)是苯甲酰甲酸酯和硫胺素的共轭物,会损失CO 2形成经典的Breslow中间体(2),其预期结果是形成苯甲醛的硫胺素共轭物(3)。出乎意料的是,观察到2与预期的质子化竞争而分解为4和5,并重排为6,从而得到3。最近的报告提出质子化的替代方法来自均质化,然后是自由基为中心的过程。现在发现,为支持所提出的自由基途径而引用的光谱观察结果很可能是其他事件的结果。另一种解释是2的烯醇羟基的电离和产生的电子重组会导致CC键断裂和易于形成4、5和6的非自由基中间体。































 京公网安备 11010802027423号
京公网安备 11010802027423号