Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-04-27 , DOI: 10.1016/j.apcatb.2017.04.073 Said Laassiri , Constantinos D. Zeinalipour-Yazdi , C. Richard A. Catlow , Justin S.J. Hargreaves
|
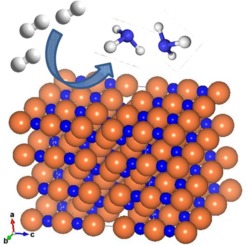
A systematic study was carried out to investigate the potential of manganese nitride related materials for ammonia production. A-Mn-N (A = Fe, Co, K, Li) materials were synthesised by nitriding their oxide counterparts at low temperature using NaNH2 as a source of reactive nitrogen. The reactivity of lattice nitrogen was assessed using ammonia synthesis as a model reaction. In the case of Mn3N2, limited reactivity was observed and only 3.1% of the available lattice nitrogen was found to be reactive towards hydrogen to yield ammonia while most of the lattice nitrogen was lost as N2. However, the presence of a co-metal played a key role in shaping the nitrogen transfer properties of manganese nitride and impacted strongly upon its reactivity. In particular, doping manganese nitride with low levels of lithium resulted in enhanced reactivity at low temperature. In the case of the Li-Mn-N system, the fraction of ammonia formed at 400 °C corresponded to the reaction of 15% of the total available lattice nitrogen towards hydrogen. Li-Mn-N presented high thermochemical stability after reduction with hydrogen which limited the regeneration step using N2 from the gas phase. However, the results presented herein demonstrate the Li-Mn-N system to be worthy of further attention.
中文翻译:

氮化锰基材料作为氮转移试剂的潜力,可用于氮化学循环
进行了系统的研究,以研究氮化锰相关材料在生产氨中的潜力。A-Mn-N(A = Fe,Co,K,Li)材料是通过使用NaNH 2作为活性氮源在低温下氮化相应的氧化物而合成的。使用氨合成作为模型反应来评估晶格氮的反应性。在Mn 3 N 2的情况下,观察到有限的反应性,发现只有3.1%的可用晶格氮对氢气具有反应性,可产生氨,而大部分晶格氮则作为N 2损失掉。。但是,共金属的存在在塑造氮化锰的氮转移特性中起着关键作用,并对其反应性产生了强烈影响。特别地,用低水平的锂掺杂氮化锰导致在低温下增强的反应性。在Li-Mn-N系统的情况下,在400°C时形成的氨的比例相当于总可用晶格氮的15%对氢的反应。Li-Mn-N在用氢还原后表现出高的热化学稳定性,这限制了使用气相中的N 2的再生步骤。但是,本文给出的结果表明,Li-Mn-N体系值得进一步关注。































 京公网安备 11010802027423号
京公网安备 11010802027423号