当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silicon carboxylate derived silicon oxycarbides as anodes for lithium ion batteries†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-04-14 00:00:00 , DOI: 10.1039/c7ta01843f M. S. Tahir 1, 2, 3, 4 , M. Weinberger 1, 2, 3, 4 , P. Balasubramanian 3, 4, 5 , T. Diemant 3, 4, 6, 7 , R. J. Behm 1, 2, 3, 4, 6 , M. Lindén 3, 4, 7, 8 , M. Wohlfahrt-Mehrens 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-04-14 00:00:00 , DOI: 10.1039/c7ta01843f M. S. Tahir 1, 2, 3, 4 , M. Weinberger 1, 2, 3, 4 , P. Balasubramanian 3, 4, 5 , T. Diemant 3, 4, 6, 7 , R. J. Behm 1, 2, 3, 4, 6 , M. Lindén 3, 4, 7, 8 , M. Wohlfahrt-Mehrens 1, 2, 3, 4, 5
Affiliation
|
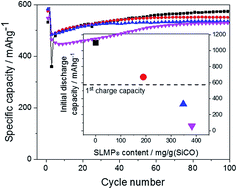
A novel and facile procedure was developed to synthesize silicon oxycarbides which are suitable anode materials for lithium ion batteries. Silicon tetraacetate was thoroughly mixed with varying amounts of citric acid and the mixtures were thermally treated at lower temperatures of up to 250 °C for several hours. The mixtures start to melt at around 150 °C. Acetic acid and acetic anhydride may then continuously be removed via distillation until the mixture starts to solidify. The degree of substitution may be controlled by the amount of citric acid. The resulting solids were then carbonized under an inert gas atmosphere at a temperature of 1000 °C. Black solids were obtained whose chemical composition and morphology were characterized in detail. X-ray diffraction (XRD) confirmed the amorphous nature of the materials. With X-ray photoelectron spectroscopy (XPS) we were able to analyze the chemical environment of the silicon atoms in more detail. Compared to fumed silica the Si 2p detail spectra show another peak at lower binding energy, which can be attributed to oxycarbide species. XPS also allowed us to determine the O/Si ratio from which a chemical formula for the SiCO materials could be derived. The materials also contain a significant amount of free carbon, which improves electrical conductivity and thus the electrochemical properties. In order to elucidate the potential of the materials as anodes for lithium ion batteries, galvanostatic charge/discharge measurements were conducted. Within the cut off potentials of 0.005–1.5 V, the materials showed high capacities of up to 590 mA h g−1 at a current of 50 mA g−1. To overcome the irreversible capacities in this system, a prelithiation approach via spray coating with stabilized lithium metal powders (SLMP®) was successfully applied. The electrodes, fabricated with lithiated polyacrylic acid (LiPAA) as binder, maintained the cycling characteristics of their non-lithiated counterparts, even if the anode was almost completely lithiated.
中文翻译:

羧酸硅衍生的碳氧化硅用作锂离子电池的阳极†
开发了一种新颖且容易的方法来合成碳氧化硅,该碳氧化硅是锂离子电池的合适负极材料。将四乙酸硅与不同量的柠檬酸充分混合,然后将混合物在最高250°C的较低温度下热处理数小时。混合物在约150°C时开始熔化。然后可以通过以下方法连续除去乙酸和乙酸酐蒸馏直至混合物开始固化。取代度可以通过柠檬酸的量来控制。然后将所得固体在惰性气体气氛下于1000℃下碳化。得到黑色固体,其化学组成和形态得到了详细表征。X射线衍射(XRD)证实了材料的无定形性质。借助X射线光电子能谱(XPS),我们能够更详细地分析硅原子的化学环境。与气相二氧化硅相比,Si 2p详细光谱在较低的结合能下显示了另一个峰,这可归因于碳氧化物。XPS还使我们能够确定O / Si比,从中可以得出SiCO材料的化学式。这些材料还包含大量的游离碳,这改善了电导率,从而改善了电化学性能。为了阐明该材料作为锂离子电池阳极的潜力,进行了恒电流充电/放电测量。在0.005–1.5 V的截止电位范围内,该材料显示出高达590 mA hg的高容量-1在50 mA g -1的电流下。为了克服该系统中不可逆的能力,成功地采用了通过喷涂稳定锂金属粉末(SLMP®)的预锂化方法。即使阳极几乎完全锂化,使用锂化聚丙烯酸(LiPAA)作为粘合剂制造的电极仍保持其非锂化对应物的循环特性。
更新日期:2017-04-14
中文翻译:

羧酸硅衍生的碳氧化硅用作锂离子电池的阳极†
开发了一种新颖且容易的方法来合成碳氧化硅,该碳氧化硅是锂离子电池的合适负极材料。将四乙酸硅与不同量的柠檬酸充分混合,然后将混合物在最高250°C的较低温度下热处理数小时。混合物在约150°C时开始熔化。然后可以通过以下方法连续除去乙酸和乙酸酐蒸馏直至混合物开始固化。取代度可以通过柠檬酸的量来控制。然后将所得固体在惰性气体气氛下于1000℃下碳化。得到黑色固体,其化学组成和形态得到了详细表征。X射线衍射(XRD)证实了材料的无定形性质。借助X射线光电子能谱(XPS),我们能够更详细地分析硅原子的化学环境。与气相二氧化硅相比,Si 2p详细光谱在较低的结合能下显示了另一个峰,这可归因于碳氧化物。XPS还使我们能够确定O / Si比,从中可以得出SiCO材料的化学式。这些材料还包含大量的游离碳,这改善了电导率,从而改善了电化学性能。为了阐明该材料作为锂离子电池阳极的潜力,进行了恒电流充电/放电测量。在0.005–1.5 V的截止电位范围内,该材料显示出高达590 mA hg的高容量-1在50 mA g -1的电流下。为了克服该系统中不可逆的能力,成功地采用了通过喷涂稳定锂金属粉末(SLMP®)的预锂化方法。即使阳极几乎完全锂化,使用锂化聚丙烯酸(LiPAA)作为粘合剂制造的电极仍保持其非锂化对应物的循环特性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号