当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preferential Solvation/Hydration of α-Chymotrypsin in Water–Acetonitrile Mixtures
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-04-26 00:00:00 , DOI: 10.1021/acs.jpcb.7b01632 Vladimir A. Sirotkin 1 , Alexandra A. Kuchierskaya 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-04-26 00:00:00 , DOI: 10.1021/acs.jpcb.7b01632 Vladimir A. Sirotkin 1 , Alexandra A. Kuchierskaya 1
Affiliation
|
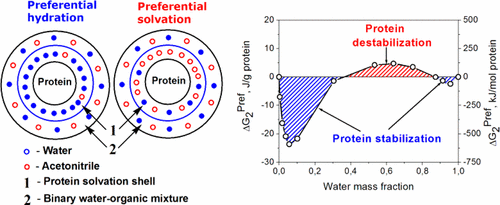
The aim of our study is to monitor the preferential hydration/solvation of the protein macromolecules at low and high water content in water–organic mixtures. Our approach is based on the analysis of the absolute values of the water/organic solvent sorption. We applied this approach to estimate the protein stabilization/destabilization due to the preferential interactions of α-chymotrypsin with water–acetonitrile mixtures. At high water content, α-chymotrypsin is preferentially hydrated. At the intermediate water content, the preferential interaction changed from preferential hydration to preferential binding of acetonitrile. From infrared spectra, changes in the structure of α-chymotrypsin were determined through an analysis of the structure of the amide I band. Acetonitrile augments the intensity of the 1626 cm–1 band assigned to the intermolecular β–sheet aggregates. At low water content, the protein is in a glassy (rigid) state. The H-bond accepting acetonitrile molecules are not effective in solvating the dehydrated protein molecules alone. Therefore, the acetonitrile molecules are preferentially excluded from the protein surface, resulting in the preferential hydration. Advantages of our approach: (i) The preferential interaction parameters can be determined in the entire range of water content in water–organic mixtures. (ii) Our approach facilitates the individual evaluation of the Gibbs energies of water, protein, and organic solvent.
中文翻译:

水-乙腈混合物中α-胰胰蛋白酶的优先溶剂化/水合
我们研究的目的是监测水-有机混合物中低和高水含量时蛋白质大分子的优先水合/溶剂化。我们的方法基于对水/有机溶剂吸附的绝对值的分析。由于α-胰凝乳蛋白酶与水-乙腈混合物的优先相互作用,我们应用这种方法来估计蛋白质的稳定/去稳定作用。在高水含量下,α-胰凝乳蛋白酶优先被水合。在中间水含量下,优先相互作用从优先水合变为乙腈的优先结合。通过分析酰胺I带的结构,从红外光谱确定α-胰凝乳蛋白酶的结构变化。乙腈可增强1626 cm –1的强度分配给分子间β-折叠聚集体的条带。在低水含量下,蛋白质处于玻璃态(刚性)状态。氢键接受的乙腈分子不能有效地单独溶解脱水的蛋白质分子。因此,乙腈分子优先从蛋白质表面排除,导致优先水合。我们方法的优势:(i)可以在水-有机混合物的整个含水量范围内确定优先相互作用参数。(ii)我们的方法有助于对水,蛋白质和有机溶剂的吉布斯能量进行单独评估。
更新日期:2017-04-26
中文翻译:

水-乙腈混合物中α-胰胰蛋白酶的优先溶剂化/水合
我们研究的目的是监测水-有机混合物中低和高水含量时蛋白质大分子的优先水合/溶剂化。我们的方法基于对水/有机溶剂吸附的绝对值的分析。由于α-胰凝乳蛋白酶与水-乙腈混合物的优先相互作用,我们应用这种方法来估计蛋白质的稳定/去稳定作用。在高水含量下,α-胰凝乳蛋白酶优先被水合。在中间水含量下,优先相互作用从优先水合变为乙腈的优先结合。通过分析酰胺I带的结构,从红外光谱确定α-胰凝乳蛋白酶的结构变化。乙腈可增强1626 cm –1的强度分配给分子间β-折叠聚集体的条带。在低水含量下,蛋白质处于玻璃态(刚性)状态。氢键接受的乙腈分子不能有效地单独溶解脱水的蛋白质分子。因此,乙腈分子优先从蛋白质表面排除,导致优先水合。我们方法的优势:(i)可以在水-有机混合物的整个含水量范围内确定优先相互作用参数。(ii)我们的方法有助于对水,蛋白质和有机溶剂的吉布斯能量进行单独评估。































 京公网安备 11010802027423号
京公网安备 11010802027423号